Abstract
Introduction:MDS are characterized by dysplastic blood cell production and anemia, neutropenia, and thrombocytopenia. Anemia may be managed by red blood cell transfusions, but risks of infection, iron overload, and end-organ damage can limit use. Disease progression is defined, in part, by dependence on transfusions. Active treatment with chemotherapy or biotherapy directed at the underlying dysplastic cells can reduce transfusion requirements, although optimum timing for initiation is not fully understood.
Methods:This retrospective cohort study analyzed 2006-2012 linked Surveillance, Epidemiology, and End Results (SEER) registry and Medicare claims to estimate the impact of treatment timing on the likelihood of reaching transfusion independence (TI) among transfusion-dependent (TD) lower-risk MDS patients (International Classification of Diseases [ICD]-O-3: 9980-9989) ≥ 60 years of age receiving active treatment (azacitidine, decitabine, or lenalidomide). The start of TD (≥ 1 blood transfusion in each of 2 consecutive 8-week periods, without a transfusion-free gap of ≥ 56 days) for the first time between 1/1/2007 and 12/31/2011 was defined as the index date. Patients not continuously enrolled for 12 months before index (baseline) or 6 months after (follow-up) were excluded. Patients were followed from index either until achieving TI or to the end of follow-up in the database. We compared early (treatment initiated ≤ 3 months from index date) vs late (> 3 months) initiators of active treatment for all outcomes. The primary outcome, TI (defined as a ≥ 56-day transfusion-free gap), was examined using multivariate Cox regression and Kaplan-Meier survival estimates. Cox models adjusted for patient demographics, treatment exposure threshold (≥ 3 prescription fills for lenalidomide or ≥ 6 administration cycles of hypomethylating agents [HMAs], based on clinical trial experience for response), and other disease characteristics such as MDS pathological categories. Components of the previously published SEER-Medicare MDS Risk Score (SMMRS) were used to categorize pathological or disease severity.
Results:We identified 508 actively treated TD patients with MDS (351 early and 157 late treatment initiators). Median age was 77 years (range 38-91); 45.5% were female; and all US geographic regions were represented. Six percent of patients had deletion 5q [del(5q)] syndrome (6.3%). Disease severity varied between early and late initiators (P < 0.001), although in each group > 70% of patients had MDS of a higher severity. For early treatment initiators, mean time ± standard deviation to active treatment was 28 ± 25 days, vs 187 ± 114 days for late treatment initiators. Proportions of patients receiving an HMA (azacitidine 56.1%, decitabine 22.8%) or lenalidomide (21.1%) were similar for early vs late initiators (P = 0.221). Most patients (65.4%) received an erythropoiesis-stimulating agent (ESA) during their TD period; however, a smaller proportion of early initiators used ESAs vs late initiators (61.5% vs 73.9%; P = 0.007). A similar proportion of early and late initiators met the minimum treatment exposure threshold for assessing response to their chosen active treatment (≥ 6 cycles of HMA or ≥ 3 cycles of lenalidomide) (37.4%; P = 0.886).
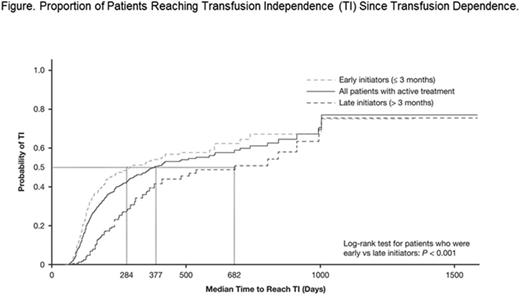
The median time to reach TI was significantly shorter in early initiators vs late initiators (284 days vs 682 days; P < 0.001) (Figure). The unadjusted rate of achieving TI was higher in early vs late initiators (0.72 per patient year [PPY] vs 0.40 PPY). This difference was observed irrespective of the minimum treatment exposure threshold being met. The proportion of patients reaching TI over time was higher in early initiators vs late initiators at nearly all time points (P < 0.001; Figure). In adjusted analyses, early treatment initiation was a significant predictor of reaching TI during the study period vs late initiation (hazard ratio [HR] 1.69; P < 0.001). Patients who met the minimum treatment exposure were also more likely to reach TI vs those who did not (HR 2.12; P < 0.001).
Conclusions:Patients with TD MDS who began active treatment ≤ 3 months of becoming TD achieved TI earlier and at a higher rate than those who initiated active treatment after > 3 months. Further study of MDS clonal evolution is needed to understand how early vs late treatment exposure impacts the subclonal architecture of MDS.
Reddy:Celgene Corporation: Other: Other I am an employee of the Partnership for Health Analytic Research, LLC, a health services research firm paid by Celgene to conduct the research described in this abstract.. Chang:Celgene Corporation: Other: Other I am an employee of the Partnership for Health Analytic Research, LLC, a health services research firm paid by Celgene to conduct the research described in this abstract.. Papoyan:Celgene Corporation: Other: Other I am an employee of the Partnership for Health Analytic Research, LLC, a health services research firm paid by Celgene to conduct the research described in this abstract.. Broder:Celgene Corporation: Other: Other I am an employee of the Partnership for Health Analytic Research, LLC, a health services research firm paid by Celgene to conduct the research described in this abstract.. McGuire:Celgene Corporation: Employment, Equity Ownership. Binder:Celgene Corporation: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.