Abstract
Introduction: ALL is a rare disease. Older adults with ALL have a markedly poor prognosis and higher mortality that may be attributable in part to greater comorbidity and lower use of intensive therapeutic options. Limited data are available about the real-world treatment patterns of elderly ALL patients in the US including their likelihood of receiving chemotherapy. In this study, we described treatment patterns in a population-based cohort of older adults with ALL.
Methods: Using 100% Medicare ALL data from 2007-2012, we identified adults age ≥ 66 years diagnosed with ALL from 2008-2011 who were continuously enrolled in Medicare Part A and B for 12 months before ALL diagnosis (baseline period) and had Part D coverage for ≥ 30 days after diagnosis. Presence of ALL was defined by ≥1 Part A inpatient (IP)/skilled nursing facility (SNF)/home health agency (HHA)/hospice (HS) or ≥2 Part A outpatient (OP)/Part B (PB) claims carrying an ALL diagnosis code on different dates in any 2-month interval. The date of ALL diagnosis was defined as the earlier date of the 1st IP/SNF/HHA/HS claim or the 2nd of 2 OP/PB claims carrying an ALL code. Baseline comorbidity level was defined using a modified Charlson Comorbidity Index (CCI). During the follow-up period, from diagnosis to the earliest of death, disenrollment from Part A, B, and D coverage, enrollment in an HMO, or December 31, 2012, we identified chemotherapy by presence of a claim containing billing codes for a specific chemotherapy agent or chemotherapy administration; use of tyrosine kinase inhibitors (TKI) was identified by the presence of corresponding National Drug Codes within Part D pharmacy claims. We defined chemotherapy course from the first claim within 90 days of diagnosis until the last claim with a < 60-day gap between two consecutive claims for chemotherapy. Baseline patient characteristics and treatment patterns were described. Chi-square test was used to assess the differences in baseline characteristics between treatment groups. Kaplan-Meier method was used to estimate the median overall survival (OS) and the 95% confidence interval (CI) from treatment initiation.
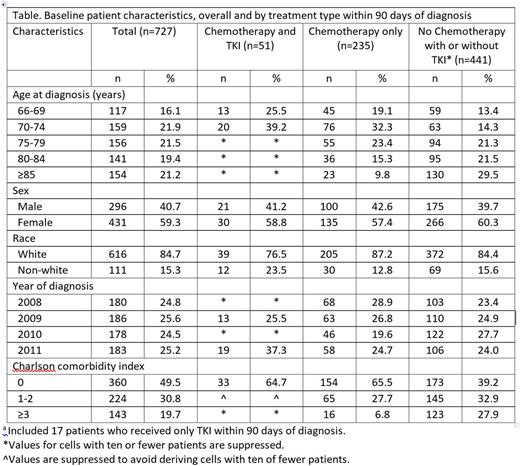
Results: Of 727 patients who met study inclusion criteria, 235 (32%) received treatment with only chemotherapy within 90 days of ALL diagnosis, 51 (7%) with chemotherapy and TKI, and 17 (2%) with only TKI. Chemotherapy-treated patients with or without TKI were younger and had lower comorbidity burden compared with patients not receiving chemotherapy (P<0.001 for age and CCI; Table). Median OS (95% CI) from treatment initiation was 10.2 (8.3-12.7) months for patients who received treatment with only chemotherapy, 18.1 (11.3-31.2) months for those with chemotherapy and TKI, and 5.8 (1.2-13.7) for those with only TKI.
Among 286 (39%) chemotherapy-treated patients regardless of TKIs, the median (interquartile range [IQR]) time to chemotherapy initiation was 3 (0-11) days. Chemotherapy was administered in 209 (73%) patients in an inpatient setting with mean (SD) length of stay 22.9 (13.9) days; of these, 90 (43%) received chemotherapy only in an inpatient setting within the first chemotherapy course. There were 177 (62%), 74 (26%), and 35 (12%) patients who received 1, 2, and ≥3 chemotherapy courses, respectively, for a total of 450 chemotherapy courses with median (IQR) course duration of 53.5 (22-137) days.
Overall, 68 (9%) patients had a TKI prescription filled within 90 days of diagnosis; of these, 19%, 32%, and 49% were aged 66-69, 70-74, and ≥ 75 years, respectively; 57% were female; and 79% were white. The median (IQR) time to first prescription fill date was 20 (12-34) days. Imatinib was more common as the first TKI agent (n=55; 81%) than dasatinib (n=13; 19%). In total, 20 (29%) patients switched TKI agents; of these, 16 (80%) switched from imatinib to dasatinib.
Conclusions: Among elderly Medicare beneficiaries diagnosed with ALL in 2008-2011, 39% were treated with chemotherapy and 9% were prescribed a TKI within 90 days of diagnosis. Patients with advanced age and higher comorbidity level were less likely to receive chemotherapy. Median OS was 10 months for patients with only chemotherapy, 18 months for patients with chemotherapy and TKI, and 6 months for patients with only TKI. These findings demonstrate the unmet need for safe and effective treatments for elderly ALL patients. Further studies assessing the comparative effectiveness and benefit-risk of treatments are warranted.
Chia:Amgen Inc: Employment, Equity Ownership. Katz:Amgen Inc: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.