Abstract

Blinatumomab is a bispecific anti-CD3/CD19 monoclonal antibody. It showed efficacy as a single agent in the treatment of relapsed/refractory Philadelphia chromosome positive acute lymphoblastic leukemia (Ph+ ALL). T-regulatory (T-reg) and T-helper cells can inhibit effector T-cells used by blinatumomab. Tyrosine-kinase inhibitors (TKI) are the basic treatment of the Ph+ ALL. To improve the long-term results of the relapsed Ph+ ALL treatment we combined blinatumomab with TKIs dasatinib or nilotinib or ponatinib.
The aim of this study was to evaluate clinical efficacy of blinatumomab + TKI combination in relapsed Ph+ ALL patients and to study T-cell and NK subpopulations kinetics in R/R-ALL patients during the treatment.
From Oct 2015 to June 2016 we treated 6 relapsed Ph+ ALL patients (5 overt hematological relapses and 1 cytogenetic relapse). Blinatumomab was administered in the dose 28 mcg/day by continuous IV infusion 28 days (4 week cycle) with 2 weeks intervals (up to 4 cycles). The dose of blinatumomab in the 1st week of the 1st cycle was 9 mcg/day. Dasatinib 140 mg/day PO started on 1 week and administered daily continuously. Nilotinib 400 mg bid PO was administered in the case of dasatinib toxicity. Ponatinib 45 mg /day PO was administered in the case of T315I ABL kinase domaine mutation. Lymphocytes subpopulations of the peripheral blood (T-helper CD3+/CD4+/CD8-, T-cytotoxic CD3+/CD4-/CD8+, T-reg CD3+/CD4+/CD25+, NK CD3-/CD56+) were studied by flow cytometry on the 1st day of every week during every blinatumomab cycle.
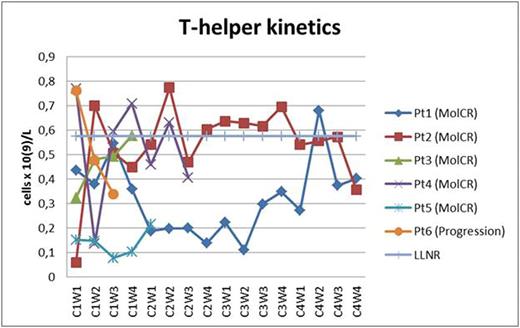
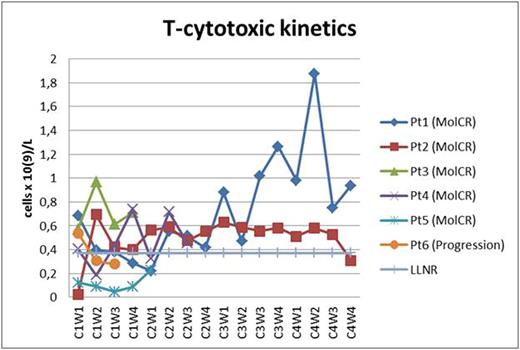
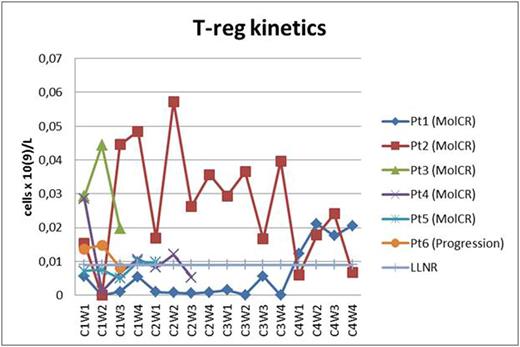
Two pts received all 4 cycles of blinatomomab + TKI dasatinib (two MolCR after 2nd cycle). Two pts received 2 cycles of blinatumomab + TKI dasatinib (two MolCR) and are continuing treatment. 1 pt with T315I mutation received 1 cycle of blinatumomab + TKI ponatinib (MolCR). 1 pt progressed during 1st cycle of blinatumomab + dasatinib which were discontinued. AlloBMT was performed in 2 pts in MolCR and 3 pts in MolCR are awaiting alloBMT. All 6 pts are alive. The pt who progressed on the 1-st cycle of blinatumomab + dasatinib was later diagnosed with T315I mutation and CR was obtained on ponatinib + dexamethasone + vincristine. In 1 pt the dasatinib-related pleural effusions and lung infiltration observed which fully regressed after 2 weeks of dasatinib interruption. Dasatinib in that pt was later replaced with nilotinib during 4th cycle and so on. Hypogammaglobulinemia was common and corrected with intravenous human normal immunoglobulin replacement. No neurological toxicity observed. As a result, 5 MolCR were obtained in 6 pts who received blinatumomab + TKI except in 1 pt with T315I mutation who progressed on blinatumomab + dasatinib. In all pts T-helper population was about low limit of normal range or below it (Fig. 1). In 4 of 5 responded pts the restoration of cytotoxic T-cell subpopulation was observed (Fig. 2). In 4 of 5 responded pts the 2nd week T-reg dropping occurred (Fig. 3). In all of responded pts NK population returned to normal range.
The blinatumomab + TKI combination is effective in relapsed Ph+ ALL. The MolCR obtaining is possible without glucocorticoid treatment. The combination has acceptable toxicity. The T-reg subpopulation depletion on 2 week and depleted pool of T-helper are correlated with MolCR obtaining during blinatumomab + TKI treatment. The T cell cytotoxic and NK lymphocyte subpopulation restoring possibly reflects normal hemopoiesis establishing in MolCR settings in effectively treated pts.
T-helper subpopulation kinetics (Pt - patient; C - cycle; W - week; LLNR - lower limit of normal range).
T-helper subpopulation kinetics (Pt - patient; C - cycle; W - week; LLNR - lower limit of normal range).
T-cytotoxic subpopulation kinetics (Pt - patient; C - cycle; W - week; LLNR - lower limit of normal range).
T-cytotoxic subpopulation kinetics (Pt - patient; C - cycle; W - week; LLNR - lower limit of normal range).
T-regulatory subpopulation kinetics (Pt - patient; C - cycle; W - week; LLNR - lower limit of normal range).
T-regulatory subpopulation kinetics (Pt - patient; C - cycle; W - week; LLNR - lower limit of normal range).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract