Abstract
To date, there are no effective strategies to treat patients with a particularly lethal form of diffuse large B-cell lymphoma (DLBCL) that over-express both B-cell lymphoma gene-2 (BCL2) and cellular-myelocytomatosis (MYC) oncogenes. Here, we provide a novel approach for simultaneously targeting BCL2 and MYC directly through secondary DNA structures. These structures act as molecular switches, turning gene expression on or off, and serve as scaffolds for protein and small molecule interaction. Previously identified compounds IMC-76 and GQC-05, recognize the BCL2 or MYC promoter region DNA structures, respectively, and independently lower mRNA and protein expression of each oncogene.For utility in sensitizing aggressive lymphoid tumors that concurrently dual express BCL2 and MYC, we examined the efficacy ofIMC-76 and GQC-05 in the presence and absence of a standard chemotherapy, cyclophosphamide (CPA)to inhibit tumor growth in a severe combinedimmunodeficient U2932 DLBCLxenograft mouse model.
Unlike IMC-76, effects of GQC-05 in a xenograftmouse model are unknown; therefore, our initial in vivo studies involved GQC-05 alone and with IMC-76 in combination with CPA to determine the concentration for MYCdown-regulation and a maximum tolerated dose (MTD). At 2.5 and 5 mg/kg GQC-05 with 50 mg/kg CPA, we observed no effect on mean mouse weight and while the 5 mg/kg GQC-05-CPA combination group exhibited some mouse death, LD50 was not reached. Tumors from mice treated with GQC-05 and CPA were compared to diluent or CPA alone treated mice for MYC mRNA level and found to have significantly less MYC expression. As expected, there was no effect on BCL2 mRNA levels. Next, we carried over the 5 mg/kg GQC-05 to a subsequent in vivo experiment where we also tested a 10 mg/kg GQC-05 dose and included IMC-76 at 10 mg/kg, a previously established efficacious dose, to determine if we would reach an MTD, but still retain the repression of MYC. While the mice were able to tolerate the 5 mg/kg GQC-05 dose when IMC-76 and/or CPA was added to the treatment regimen, the LD50 was reached and surpassed in mice treated with the 10 mg/kg of GQC-05 combinations. However, both concentrations of GQC-05 when co-treated with IMC-76 and CPA resulted in a notable decrease of tumor MYC expression relative to untreated and CPA only treated mice as well as a detectable inhibition of BCL2.
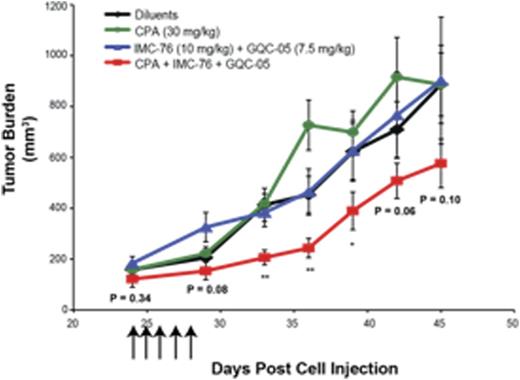
We then conducted a follow-up study to evaluate effects on tumor burden using concentrations of GQC-05 below the LD50, 5 mg/kg and 7.5 mg/kg, and also reduced the dose of CPA to 30 mg/kg. The co-treatment of mice with IMC-76 (10 mg/kg) and GQC-05 (7.5 mg/kg) in combination with CPA delayed tumor growth and decreased tumor size by 50%, 46%, and 37% at 9, 12, and 15 days post-first-day drug administration compared to the diluent and 50%, 67%, and 44% smaller tumors relative to CPA only treated mice (Figure 1). Overall, there was a prominent decrease in tumor burden in these mice as determined by area under the curveand the tumors displayed a lower expression of BCL2 and MYC at both the mRNA and protein levels. Interestingly, tumors of mice that received IMC-76 and GQC-05 also showed knock-down of BCL2 and MYC, but no effect on tumor burden unless CPA was present indicating these transcriptional inhibitors act as chemo-sensitizers. None of the various drug regimens resulted in a significant loss of mouse weight and while survival for GQC-05 7.5 mg/kg treated mice was reduced, LD50 was not reached.
These are the first studies to demonstrate two differentpromoterDNA secondary structures can be targeted at the same time for direct transcriptional inhibition that leads tochemo-sensitization and slowed tumor growth in mice.Our findings demonstrate the potential utility of a dual-targeted, precision medicine-based strategy to improve the response of DLBCL patients to current chemotherapy and overcome resistant disease.
Tumor burden of DLBCL xenograft mice
Gokhale:Tetragene: Equity Ownership. Hurley:Tetragene: Consultancy, Equity Ownership. Rimsza:NCI/NIH: Patents & Royalties: L.M. Rimsza is a co-inventor on a provisional patent, owned by the NCI of the NIH, using Nanostring technology for determining cell of origin in DLBCL..
Author notes
Asterisk with author names denotes non-ASH members.