Abstract
Background:
Ibrutinib, a first-in-class, once-daily oral inhibitor of Bruton's tyrosine kinase (BTK), is indicated by the US FDA for the treatment of patients with chronic lymphocytic leukemia/small lymphocytic lymphoma, including patients with deletion 17p, patients with mantle cell lymphoma who have received at least 1 prior therapy, and patients with Waldenström's macroglobulinemia. In addition to blocking B-cell activation via inhibition of BTK signaling, ibrutinib was reported to modulate immune function by driving TH1 response through IL-2-inducible T-cell kinase (Dubovsky, Blood 2013). Ibrutinib was also shown to reprogram macrophages toward a TH1 phenotype that fostered CD8+ T-cell cytotoxicity in pancreas ductal adenocarcinoma-bearing mice (Gunderson, Cancer Discov 2016). Both the direct and indirect effects on TH1 response may play a role in the therapeutic efficacy of ibrutinib in models of lymphoma and solid tumors (Sagiv-Barfi, PNAS 2015). Another essential component of the immune system with a role against cancer is natural killer (NK) cells involved in the recognition and elimination of tumor cells. In this study, we evaluated the effects of ibrutinib on NK cell-mediated cytotoxicity in mouse models of B-cell lymphoma.
Methods:
Experiments in TMD8 xenograft models were performed in CB17.SCID and NSG mice, and experiments in an A20 syngeneic model were performed in BALB/c mice. Mice were orally administered ibrutinib (once daily) for a total duration of 2 weeks starting from the time tumors reached a volume of 100-150 mm3. Tumor BTK occupancy was determined by a gel-based probe assay 4 hours after the last dose of ibrutinib, and was normalized to the total BTK level. NK cell-mediated cytotoxicity was also evaluated in mice carrying X-linked spontaneous mutation in BTK, Btk(xid) mice (009361; Jackson Laboratory). For the NK cell cytotoxicity assay, mouse splenocytes were added to PKH67-labeled YAC-1 cells at different effector-to-target ratios and incubated at 37°C for 4 hours. Propidium iodide was added and flow cytometry was performed to determine target cell viability. Human or mouse cytokines/chemokines were quantified using MILLIPLEX® MAP Kit (HCYTMAG-60K-PX38 and MCYTMAG-70K-PX32).
Results:
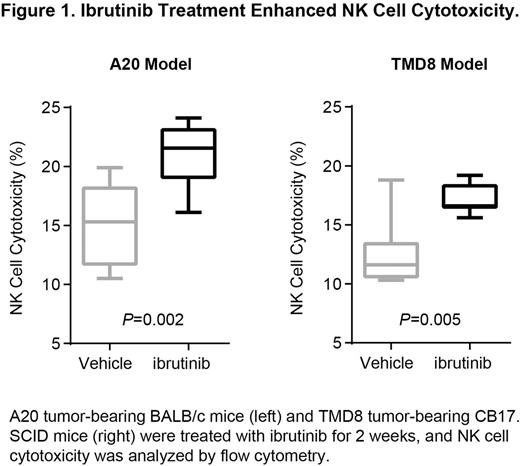
In TMD8 ABC-DLBCL models, tumor suppression after ibrutinib treatment was only observed in CB17.SCID mice but not in NSG mice despite tumor BTK occupancy of >90% in both models. As these 2 strains of mice differ in their NK cell profile (NSG mice lack mature T, B, and NK cells whereas CB17.SCID mice are severely deficient in T and B cells), we were interested in studying the immune modulation function of ibrutinib on NK cells. Interestingly, elevated NK cell cytotoxicity was seen in Btk(xid) mice compared to Btk wild-type mice, suggesting a potential role of BTK depletion in NK cell function. Treatment with ibrutinib increased NK cell-mediated cytotoxicity in both syngeneic A20 B-cell lymphoma and TMD8 xenograft models (Figure 1), but did not affect NK cell population (% of NKp46+ or CD3-CD49b+). A20 tumor-bearing mice had lower NK cell cytotoxicity compared to non-tumor-bearing mice, suggesting suppression of NK cell function by tumors. In addition, ibrutinib reduced tumor-derived cytokines IL-6 and IL-10, which are negative regulators of NK cells. IFN-gamma secreted by nontumor cells was increased in the sera of CB17.SCID but not in NSG mice after ibrutinib treatment, providing further evidence of the increased NK function with ibrutinib. The detailed mechanisms of modulation of NK cell function by ibrutinib is currently under investigation.
Conclusions:
We report herein that ibrutinib enhanced NK cell-mediated cytotoxicity in mouse models of B-cell lymphomas. In addition to its direct effect on BTK inhibition in tumor cells, these data provide further evidence of the immune modulation function of ibrutinib. The role of ibrutinib in NK cell activation as observed in the current study may further expand the potential application of ibrutinib therapy.
Kuo:Pharmacyclics, LLC, an AbbVie Company: Employment, Other: Travel, Accommodations, and Expenses, Patents & Royalties: Pharmacyclics, LLC, an AbbVie Company; AbbVie: Equity Ownership. Hsieh:Pharmacyclics, LLC, an AbbVie Company: Employment. Whang:Pharmacyclics, LLC, an AbbVie Company: Employment; AbbVie: Equity Ownership. Huang:Juno: Equity Ownership; Pharmacyclics, LLC, an AbbVie Company: Employment; Five Prime: Equity Ownership; Merrimack: Equity Ownership. Sirisawad:AbbVie: Equity Ownership; Pharmacyclics, LLC, an AbbVie Company: Employment. Chang:AbbVie: Equity Ownership; Pharmacyclics, LLC, an AbbVie Company: Employment, Patents & Royalties: Pharmacyclics, LLC, an AbbVie Company.
Author notes
Asterisk with author names denotes non-ASH members.