Abstract
BACKGROUND: GZL (B-cell lymphoma, unclassifiable, with features intermediate between DLBCL andcHL) was first described in 2005 and included in the 2008 WHO classification. The majority of cases present withmediastinal disease and share features withcHL and primarymediastinal large B-cell lymphoma (PMBCL). Non-mediastinal lymphomas with similar features have also been reported. Due to the relative rarity and the diagnostic complexity of this disease, data on GZL are limited and further description of this entity is desired.
METHODS: Clinical data from cases originally diagnosed as GZL were collected from 15 academic centers across the United States and Canada (Evens et al. Am J Hematol, 2015). In an attempt to further characterize the diagnostic features and clinical correlations, 73 cases (including 62 cases from the aforementioned series and 11 subsequently collected cases) were obtained and submitted for central pathology review using criteria of the 2016 revisedWHO classification. All diagnostic samples were evaluated with a panel comprising CD20, CD79a, PAX5, OCT2, BCL6, MUM1, CD30, CD15, CD3 and EBV by in situ hybridization (EBER). Beyond the tumor cellimmunoprofile, diagnostic criteria included: tumor cell density and morphology, necrosis, and the microenvironment. Five cases were rejected for insufficient material/technical issues. Collectively, 68 cases were evaluated by 5 experthematopathologists and consensus diagnosis was reached at multi-headed scope review. Additionally, clinical data were obtained to analyze patient (pt) characteristics and disease outcomes.
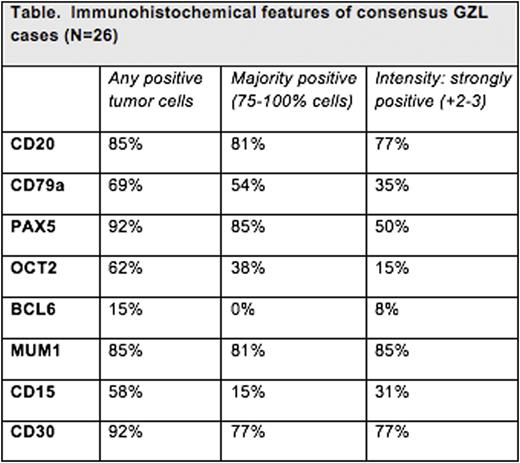
RESULTS: Of 68 cases given an original diagnosis of GZL from academic institutions, only 26 cases (38%) were confirmed as GZL on consensus review. Pt characteristics of these 26 GZL cases included: 15M/11F; median age 37 years (range 19-72); 42% B symptoms; 61% anemia; 35% increased LDH; and 33% with hypoalbuminemia. 11/26 (42%) biopsies were mediastinal in origin, and in an additional 4 cases, a mediastinal mass was present clinically; 11 (42%) had only peripheral lymphadenopathy/disease (ie, non-mediastinal). 60% of pts had stage I/II disease with 16% having stage IV. GZL cases were characterized by high tumor cell density and paucity of a mixed inflammatory background (both contrary as seen in cHL). The immunohistochemical profiles of the 26 consensus GZL cases are noted in the Table. Notably, only 1 GZL case was EBV positive. 42/68 (62%) of the original cases were reclassified as follows: nodular sclerosis (NS)cHL, n=27 [n=10 of which werecHL, NS grade 2 (cHL-NS2)] and one lymphocyte-richcHL (LRCHL), n=1; DLBCL NOS, n=4; PMBCL, n=2; nodular lymphocyte predominant Hodgkin lymphoma (NLPHL), n=3; EBV positive LBCL, n=3; and B-cell lymphoproliferative disorder, n=1. Most cases ofcHL diagnosed as GZL had strong CD20 expression. Further, cHL-NS2 was often misdiagnosed as GZL usually due to confluent growth of lacunar cells. Clinically, therapy received and outcomes were available for 25 of 26 of the aforementioned consensus GZL cases; the overall response rate (ORR) for these consensus confirmed GZL cases was 64% (complete remission (CR) 48%) with 28% ofpts experiencing primary refractory disease to frontline therapy. Relapse rates by primary chemotherapy regimen for thesepts were: ABVD 5/6 (83%); CHOP 7/17 (41%); and EPOCH 1/2 (50%). Among consensus GZLpts with relapsed disease, 71% underwent autologous SCT. With a median follow-up of 40 months, the 3-year PFS was 44% with 3-year OS of 90% for the consensus GZL pts. Among all other cases that were reclassified to a non-GZL diagnostic entity, the ORR was 75% (CR 67%) with 3-year PFS and OS rates of 52% and 80%, respectively. This included a relapse rate of 86% amongpts with cHL-NS2.
CONCLUSIONS: Accurate diagnosis of GZL remains challenging. Relative rarity of the cases and overlap withcHL, especially thecHL-NS variant with lymphocytic depletion and confluent lacunar cells (also known as cHL-NS2), contribute to this difficulty. Diagnosis should be based on integration of architectural, cytological andimmunophenotypic features. In addition, relapse rates are high with standard chemotherapy regimens, especially ABVD-based therapy. Enhanced biologic understanding and improved therapeutic strategies are needed for GZL.
Evens:Takeda: Other: Advisory board. Abramson:Abbvie: Consultancy; Gilead: Consultancy; Seattle Genetics: Consultancy; Kite Pharma: Consultancy. Fenske:Celgene: Honoraria; Millennium/Takeda: Research Funding; Pharmacyclics: Honoraria; Seatle Genetics: Honoraria. Friedberg:Bayer: Honoraria, Other: Data Safety Monitoring Board. Blum:Pharmacyclics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.