Abstract
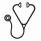
Background: Myelofibrosis (MF) in primary myelodysplastic syndromes (MDS) is a distinct clinicopathological entity from primary myelofibrosis. In the absence of therapy, it has been shown to be an independent predictor of worse overall survival (OS) and leukemia-free survival (LFS) in both low and high risk MDS (Della Porta, J. Clin. Oncol, 2009). However, there are conflicting reports as to whether this applies to higher-risk patients treated with azacitidine, the recommended first line therapy in this group (Fu, Mod. Pathol, 2014).
Methods: 93 consecutive patients from our centre treated with azacitidine for MDS or CMML between 2008 and present were identified from a national prospective MDS registry. The pre-treatment marrows of 53 patients were available for independent and retrospective MF scoring by a hematopathologist (MJ) using both the European Consensus and Bauermeister criteriafor myelofibrosis grading. Qualitative fibrosis status of either none/mild or increased/marked were extracted from original reports in 8 additonal patients for which marrows were not available for hematopathologic review. The remaining patients were excluded due to: >20% blasts pre-treatment (1), lack of pre-treatment bone marrows (20), lack of fibrosis status indicated in original report and marrow not available for review (10), and lack of response data (1). Those with European Consensus MF <2 or qualitatively mild/none were compared to those with MF >or= 2 or qualitatively increased/marked. Patients were also compared as Bauermeister grades 1-2 or mild/none vs. Bauermeister grades 3-4 or increased/marked.
Results: Median age of the 61 patients was 72 years and 74% were male. According to WHO classification, 2 had del(5q) syndrome, 5 RARS, 8 RCMD, 16 RAEB-1, 23 RAEB-2, 2 CMML1, 4 CMML2 and 1 MDS/MPN. Median hemoglobin level, neutrophil count, platelet count, LDH, ferritin, and marrow blast count were 91 g/L (range 64-136), 1 x109/L (0-46), 51 x109/L (2-459), 263 U/L (110-1442), 746 mcg/L (56-7983) and 7% (2-18), respectively. 58% were transfusion dependent at start of treatment. IPSS was High, Int-2, Int-1, and Low in 9%, 46%, 33%, and 4% of patients. 32 patients (53%) had European Consensus MF grades >or=2 or increased/marked fibrosis, and this was significantly (p=0.011) associated with higher pre-treatment ferritin levels (1110 vs. 532mcg/L). The increased MF group also showed trends towards younger age (68 vs 72 years), longer time from diagnosis until treatment (18.3 vs. 7.1 mos), greater transfusion dependence (69 vs. 45%), and pre-treatment LDH >250 U/L (61 vs. 42%), which did not meet statistical significance given limited sample size. Otherwise there were no significant relationships between MF score and WHO classification, IPSS/IPSSR, karyotype/cytogenetics, or other covariates. The results were similar when comparing groups by the alternative Bauermeister criteria, with increased fibrosis associated with a ferritin of >1000 mcg/L (p=0.038). After a median of 9 cycles (1-62) of azacitidine,complete remission (CR), marrow CR, and hematologic improvement (HI) was achieved in 9 (15%), 5 (8%), and 27 (44%) patients, respectively. Stable disease was seen in 29 (48%) patients. After a median follow-up of 3 years, median OS by Kaplan-Meier survival curve was 3.6 years in all treated patients and 2.7 years in IPSS Int-2/High patients. Regardless of which fibrosis grading system was used to compare patients, there were no significant differences in time to achieve best response, response rates, OS, or LFS based on pre-treatment MF grades (Figure 1).
Conclusion: In this prospective registry series of azacitidine treated MDS with retrospective, pathology reviewed semi-quantitative MF grading, the degree of myelofibrosis did not significantly impact response to azacitidine, progression to leukemia, or overall survival. However, increased fibrosis was significantly associated with higher pre-treatment ferritin values and resulted in a non-significant trend towards younger age, longer time to treatment, transfusion dependence, and higher pre-treatment LDH levels.
Wells:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Alexion: Honoraria, Membership on an entity's Board of Directors or advisory committees. Buckstein:Novartis: Honoraria; Celgene: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract