Abstract
Objective: To fully contextualize the benefit of novel agents such as daratumumab (DARA) monotherapy for the treatment of patients with heavily pre-treated and highly refractory multiple myeloma (MM), it is critical to understand the real-world outcomes of this patient population on current standard of care (SOC) therapies. The objective of this study was to perform adjusted comparisons to determine the comparative effectiveness of DARA monotherapy versus real-world SOC therapies.
Methods: Data for patients treated with DARA 16 mg/kg monotherapy were available from clinical trials MMY2002 (n=106) and GEN501 (n=42), while patients treated with SOC therapies were derived from the International Myeloma Foundation (IMF) chart review of patients with MM who had ≥3 prior lines of therapy and were double refractory to a proteasome inhibitor (PI) and an immunomodulatory drug (IMiD) (n=543) (Kumar et al., ASH 2016; submitted). The pooled DARA studies demonstrated a median overall survival (OS) of 20.1 months versus 13.0 months for SOC based on the IMF cohort (Usmani et al., Blood 2016; Kumar et al., ASH 2016; submitted). The relative treatment effect of DARA versus SOC was estimated using two adjusted comparison methodologies, propensity score matching (PSM) and multivariate Cox regression analyses. Both methodologies utilized individual patient data to compare OS. Modeled covariates for the PSM were age, gender, prior lines of therapy, albumin, and refractory status to bortezomib (BOR), carfilzomib (CAR), lenalidomide (LEN), and pomalidomide (POM). PSM was performed using caliper matching with a caliper width 25% of the standard deviation of the logit-transformed propensity score, using sampling without replacement. For the regression analysis, the covariates included in the multivariate proportional hazards regression model were age, gender, prior lines of therapy, albumin, beta-2 microglobulin, prior exposure to POM and CAR, and PI/IMiD refractory status. Clustering of observations at the treatment-line level within patients was controlled for using the robust sandwich estimate for the covariance matrix, making confidence intervals (CIs) more conservative. For both PSM and regression, statistical significance testing was performed using a two-tailed p-value of <0.05, and all comparisons between treatment groups were reported with hazard ratios (HRs) and 95% CIs.
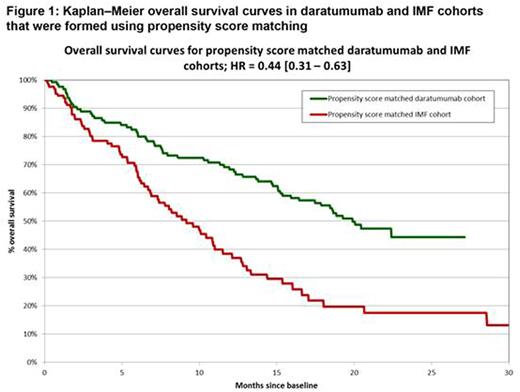
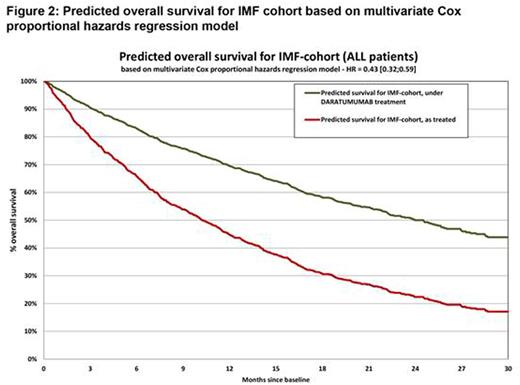
Results: Prior to PSM, imbalances between the DARA and SOC groups were significant for prior lines of therapy and proportions of patients refractory to POM, CAR, BOR, and LEN. After PSM, the DARA and SOC groups were well balanced for all covariates included in propensity score calculations. After PSM, comparisons found significant improvement in favor of DARA relative to SOC for OS (HR=0.44 [95% CI 0.31-0.63]) (Figure 1). Regression analyses revealed consistent results. After adjustment for differences in all covariates included in regression between the DARA and SOC groups, results showed significant improvement in favor of DARA compared with SOC for OS (HR=0.43 [95% CI 0.32-0.59]) (Figure 2).
Conclusions: Findings from both PSM and regression analyses were consistent and suggest that DARA is associated with significant gains in OS compared with SOC therapies for patients with heavily pre-treated and highly refractory MM.
References:
1. Usmani SZ, Weiss BM, Plesner T, Bahlis NJ, Belch A et al. (2016) Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood 128 (1): 37-44.
2. Kumar SK, et al. (2016) Natural history of relapsed myeloma, refractory to immunomodulatory drugs and proteasome inhibitors: a multicenter IMWG study. The 58th Annual Meeting of the American Society of Hematology: submitted.
Kumar:Celgene: Consultancy, Research Funding; Noxxon: Consultancy, Honoraria; Janssen: Research Funding; AbbVie: Research Funding; BMS: Consultancy; Amgen: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Sanofi: Consultancy, Research Funding; Skyline: Consultancy, Honoraria. Durie:Amgen: Consultancy; Takeda: Consultancy; Janssen: Consultancy. Su:Janssen: Research Funding. Diels:Johnson & Johnson: Employment, Equity Ownership. Hutton:Essai Canada: Consultancy; Cornerstone Research Group: Consultancy; Janssen: Research Funding. Lam:Janssen: Employment. Tetsuro:Johnson & Johnson: Equity Ownership; Janssen: Employment.
Author notes
Asterisk with author names denotes non-ASH members.