Abstract
Introduction
Bortezomib (B) has been reported to be very effective in AL amyloidosis with overall response rates (ORR) varying between 50-80%. However, no prospective data have been published from multicenter studies on B treatment in de novo patients. Previously, we have demonstrated the positive long term effect of induction therapy followed by high dose melphalan (HDM) and autologous stem cell transplantation (SCT). We therefore investigated the efficacy and safety of B-Dexamethasone (BD) induction treatment followed by HDM + SCT to improve the complete response rate (CR) in de novo AL amyloidosis patients. This report is on the first 25 patients.
Methods
The HOVON 104 trial was performed in the Netherlands, Belgium and Germany from Jan 2012 to April 2016 and started with a randomized phase III design of BD versus dexamethasone (D) induction treatment followed by HDM + SCT. Due to slow accrual the D arm closed after including 7 patients. Patients with biopsy proven AL amyloidosis, aged between 18-70 years, with detectable M-protein and/or level of involved FLC > 50 mg/L, WHO performance status 0-2, NYHA stage 1-2 and ejection fraction > 45% were included. Major exclusion criteria were symptomatic orthostatic hypotension, NT proBNP level > 5000 pg/ml, Troponin T> 0.06 ug/l, Bilirubin > 2x ULN, eGFR < 30 ml/min, CTCAE grade peripheral sensory neuropathy > grade 2 or > grade 1 with pain. Inclusion and exclusion criteria were installed both at entry and before stem cell mobilization (SCM). B was given subcutaneously 1.3 mg/m2 twice a week for 2 weeks in a 21-day cycle, D 20 mg orally on each B and the following day. HDM dosage was 200 mg/m2. Hematological responses were defined according to consensus criteria with the addition of very good partial response (VGPR), defined as the difference between involved and uninvolved FLC (dFLC) < 40 mg/L. Cardiac, renal and liver response and progression criteria were defined according to consensus criteria with addition of NT proBNP. The primary endpoint was the proportion of patients with CR at 6 months after SCT. To demonstrate improvement from 30 to 50% with 80% power, 44 eligible patients were needed and 50 patients were registered.
Results
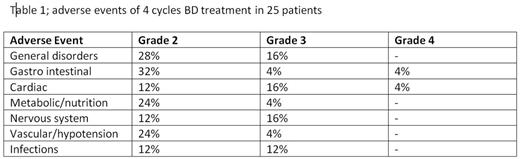
Median age was 60 years (range 26-70) and 68% were male. WHO performance status (PS) was 0-1 in 88% of patients and NYHA stage 1 in 52% and 2 in 44% of patients. Mayo cardiac risk score (2004) was I (28%), II (32%), III (36%). Organ involvement was 88% renal, 76% heart, 20% liver, 12% neurological, 4% gastrointestinal and 72% of patients had 2 or more organs involved. Bone marrow plasmacells were > 10% in 11 patients. Six of the 25 (24%) patients could not proceed to SCM. One patient due to low PS, one because of B related toxicity, two due to amyloidosis related complications and two patients died, both amyloidosis related. Of these 19 patients, 2 went subsequently off protocol because of ineligibility for HDM and one due to hematological progression. Sixteen out of 25 patients (64%) received HDM + SCT which was performed without any treatment related mortality (TRM). In total 29 SAEs were reported in 18 patients. The ORR after induction was 72% and ≥ VGPR in 56% of patients. The ORR in the 16 patients at 6 months after SCT was 75% and ≥ VGPR 63%. Median time to first response was 1 month. Intention to treat analysis demonstrated that the primary endpoint was met in 6 (24%) patients. Organ responses after induction were 9/22 renal and 3/19 heart, and at 6 months after SCT 9/14 renal and 5/13 heart. The first two BD cycles were given as planned in most patients, 80 and 70%, respectively, but doses were reduced and delayed thereafter for B in half of patients, mostly because of neurotoxicity. Mean cumulative dosage of B was 80% of planned. Also D was reduced in almost half of patients due to toxicity. The most common AEs during induction are shown in Table 1.
Conclusions
Analysis of 25 patients demonstrates that with BD treatment the dropout rate before HDM is 36% which is comparable to previous induction treatments. We therefore conclude that BD, given twice weekly, despite good efficacy, cannot prevent early amyloidosis related toxicity. The SCT procedure was without TRM. The hematological response rate is comparable to previously reported and renal response are 41% after BD and 64% at 6 months after SCT.
Trial registration www.trialregister.nl(NTR 3220), EudraCT 2010-021445-42, supported by the Dutch Cancer Society (UU 2010-4884 ) and by an unrestricted grant from Janssen-Cilag
Minnema:Celgene: Consultancy; BMS: Consultancy; Amgen: Consultancy; Jansen Cilag: Consultancy. Hazenberg:GSK: Consultancy, Speakers Bureau; Pfizer: Consultancy, Speakers Bureau. Hegenbart:Jansen Cilag: Honoraria, Other: financial support of conference participation. Ypma:Advisory Board Sanofi (Plerixafor): Membership on an entity's Board of Directors or advisory committees. Zweegman:Celgene: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Takeda: Honoraria, Research Funding. Broijl:Celgene: Honoraria; Jansen Cilag: Honoraria; Amgen: Honoraria. Sonneveld:Janssen: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Takeda: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria, Research Funding. Schoenland:Jansen: Honoraria, Other: financial support of conference participation, Research Funding; Prothena: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.