Abstract
Background: Peripheral T-cell lymphomas (PTCL) typically have a dismal outcome when treated with conventional combination chemotherapy. However, the role of HDC with ASCT in patients who attained CR1 following induction chemotherapy remains controversial.
Methods: This is a retrospective analysis performed using a prospective database of newly diagnosed PTCL patients treated at our centres from 1993 to 2015. After excluding patients with NK/T cell lymphoma, ALK-positive anaplastic large cell lymphoma, cutaneous T cell lymphoma and composite lymphomas, we identified 164 PTCL patients who were treated with curative intent. Among them, 73 patients achieved CR1 following induction chemotherapy. We compared the characteristics and outcomes of those who went on to receive further ASCT with those who did not receive further consolidation ASCT. Fisher's exact test and Student's T test was used to compare the baseline characteristics such as age, stage, international prognostic index risk group (IPI), prognostic index for PTCL (PIT), lactate dehydrogenase (LDH) and treatments between the two cohorts. Progression-free survival (PFS) and overall survival (OS) were compared using the log-rank test. Cox proportional hazard regression analysis was performed to estimate the hazard ratios (HR).
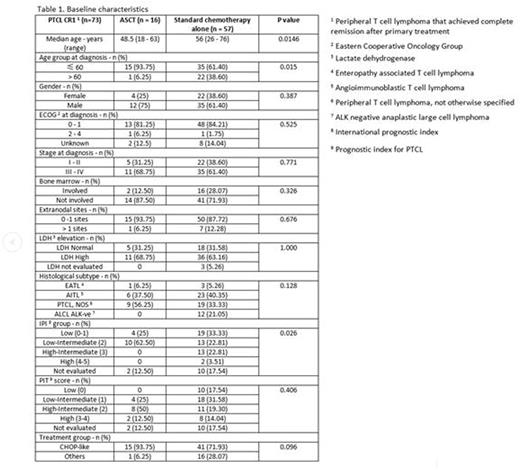
Results: Of the 164 patients treated with curative intent, the median OS of patients who achieved CR1 was not reached compared to 1.23 years among those who did not achieve CR1 with a HR of 5.24 (95% CI: 3.11-8.83, p<0.0001). The median age of the 73 PTCL patients who attained CR1 was 54 years (range: 18-76). Among them, 37% had stage I-II disease, 63% had III-IV disease, 25% had bone marrow involvement and 64% had elevated LDH. Majority of the patients (77%) received CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone)-like chemotherapy. Of the 73 patients who attained CR1 following induction chemotherapy, 16 (22%) received ASCT in CR1 while 57 (78%) received no further treatment. The baseline characteristics between these two cohorts were balanced except for age group and IPI. The median age of patients who had ASCT was 48.5 years (range: 18-63) compared to 56 years (range: 26-76) among those who did not undergo ASCT (p=0.0146). Although more patients in the non-transplanted group had higher IPI scores compared to the transplanted group, the distribution of patients across PIT risk groups was comparable (Table 1). The median OS of patients who underwent ASCT was not reached compared to 12.01 years in patients treated with conventional chemotherapy alone with a HR of 6.35 (95% CI: 0.77-52.56, p=0.09) after adjusting for age and IPI. The median PFS of patients who underwent ASCT was not reached compared to 5.18 years in conventionally treated patients with a HR of 2.33 (95% CI: 0.80-6.75, p=0.12) after adjusting for age and IPI.
Conclusion: In this retrospective study of patients with PTCL in CR1, after adjusting for age and IPI, the OS and PFS of patients who underwent ASCT do not appear to be statistically different from those who received conventional chemotherapy alone. The study suggests that patients who achieved CR1 may have tumors that are inherently biologically favorable and further consolidation therapy may not be needed, at least not for all patients. Further study is warranted to establish the true benefit of ASCT in PTCL patients achieving CR1. Uncovering the genetic differences between responders and poor responders may identify new targets for PTCL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.