Abstract
Background:
During pregnancy, vitamin B12 (B12) utilisation increases, with deficiency being associated with adverse outcomes (Murphy 2007, VanderJagt 2011). It remains unclear however at what level vitamin B12 status becomes detrimental, and the correlation with fetal harm.
Establishing B12 status during pregnancy is complicated by physiological changes such as haemodilution due to expanded blood volume, altered renal function, alterations in the vitamin B12 -binding proteins and transfer of materno-fetal B12 yet laboratories typically rely on the total abundance of B12 in serum (serum B12) asthe sole laboratory status indicator, interpreting results against reference range cut offs derived from non-pregnant populations, including men.
Serum B12 assays measure the sum of holohaptocorrin and holotranscobalamin (holoTC). Other markers of B12 status are also available and are increasingly being adopted: holoTC (active B12) accounts 6-20% of bound B12, which is the only form of B12 presented for cellular uptake and used to satisfy metabolic demand; methylmalonic acid (MMA) is a by-product of methylmalonyl-CoA metabolism, the concentration of which in serum correlates inversely with tissue B12 utilisation.
Methods:
Over a 2 monthperiod in 2015 we used an extended panel of laboratory tests consisting of serum B12, holoTC, and MMA to determine B12 status, and assess appropriateness of our assay reference ranges, in 100 pregnant women with normal renal function at the end of the first trimester.
Laboratory Analysis
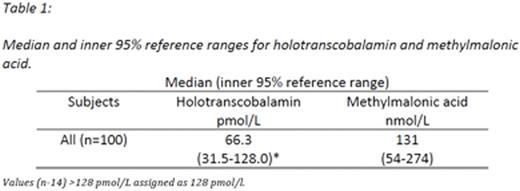
Serum B12 and holoTC were determined by an Abbott Architect analyzer. The serum B12 assay has a reference range of 187 - 883 ng/L. The holoTC assay has ²10% cross-reactivity from B12-binding proteins haptocorrin and apotranscobalamin. Values >128 pmol/L are above the dynamic assay range. In our laboratory holoTC results <25 pmol/L are defined as deficient and those >70 pmol/L as replete. Results 25-70 pmol/L are classified as indeterminate and a serum MMA assay subsequently performed (Sobczyńska-Malefora A et al 2014). We determined serum MMA using a Gerstel Multipurpose Sampler coupled to a liquid chromatography tandem mass spectrometer with electrospray ionization with results >270 nmol/L considered indicative of suboptimal B12 status (Harrington DJ 2016).
Results:
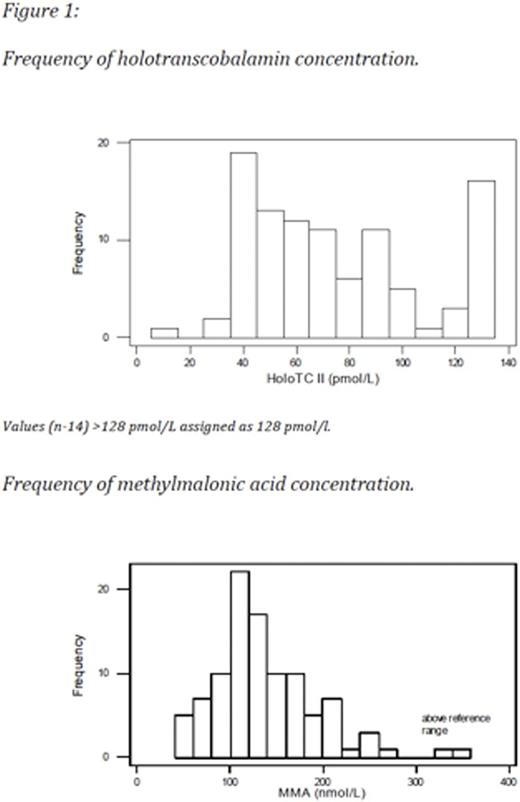
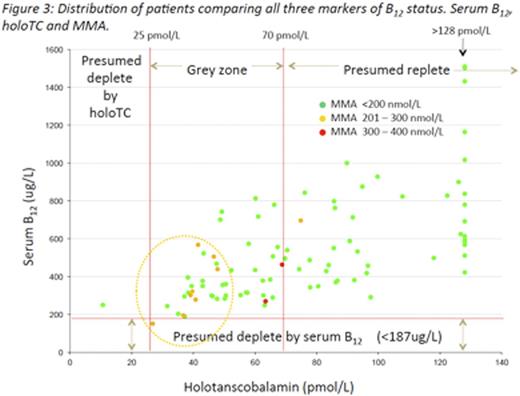
All three assays, figure 3, were performed for 92 women (insufficient sample available n=8).HoloTCand MMA results are shown in Table 1. The distribution ofholoTCand MMA concentrations are shown in Figure 1.
One woman had a serum B12 below the reference range with a corresponding indeterminate holoTC and normal MMA. This set of results suggests questionable abundance of B12 that has not translated to functional deficiency.
One woman had a holoTCconcentration <25pmol/L, however the corresponding serum B12 and MMA were not indicative of sub optimal B12 status. Fourteen women had aholoTC result above the dynamic assay range.
Two women had an elevated MMA concentration with corresponding holoTCresults indicating questionable abundance of B12 but serum B12 within our reference limit. This triad of values indicates possible emerging suboptimal B12 status that would have been overlooked if serum B12 had been used in isolation.
Discussion:
B12 requirement increases in pregnancy and diet alone is unlikely to meet requirements (Murphy et al 2007). Deficiency has a detrimental impact on maternal and infant development and therefore it is useful to explore the relationship between different laboratory markers of B12 status in pregnancy (Ramirez-Velez et al 2016, Siddiqua et al 2014). The comparison of serum B12,holoTC and MMA levels at the end of the first trimester with reference ranges derived from non-pregnant adults suggests that they are broadly applicable.
Serum B12 is the least sensitive marker and its use in isolation to assess B12 status is limiting. The application of holoTC and MMA in tandem can reveal previously missed deficient states. Using these markers two women in this small study were identified as possibly deficient although the true significance of a mildly elevated MMA in pregnancy requires further investigation. These data highlight the benefit of adopting a panel of markers ahead of a single test to assess B12 status. Identifying women at the end of the first trimester that may benefit from B12 replacement potentially protects from deficient states.
Robinson:Pharmacosmos: Honoraria; Amgen: Honoraria; Novartis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.