Abstract
Background: Chronic kidney disease (CKD) is a prevalent complication of sickle cell disease (SCD) associated with early mortality. Hemoglobinuria is a risk factor for the development of albuminuria and CKD. Currently, there are no biomarkers that predict outcome of CKD. Mass-spectrometry analysis of patient urine is a highly potent modern method for biomarker discovery. An in vitro glomerular permeability assay has been used as a non-invasive test for glomerular disease prognosis. Application of this assay to urinary samples collected before kidney disease onset provides a unique opportunity for differential proteomics of a limited number of urinary proteins. In combination with high resolution/selected ion monitoring (HR/SIM) mass-spectrometry methodology, this experimental platform provides an opportunity for biomarker discovery and validation of proteins whose concentration is too low to be detected by an immunological assay.
Objectives: We aimed to determine a biomarker of early stage kidney disease using samples collected from the patients of the Center for Sickle Cell Disease at Howard University. We then used HR/SIM method to validate this biomarker in the cohort of SCD patients with and without CKD from University of Illinois at Chicago (UIC).
Methods:Urinary protein, creatinine, albumin were measured by ELISA. The pH and specific gravity (SG) were determined by Multistix. Glomeruli were isolated from the murine kidney (FVB/N strain) and albumin permeability (Palb) activity was determined using urine samples collected from patients of the Center for Sickle Cell Disease, Howard University. Mass-spectrometry analysis was performed and Protein Discovery 1.4 and SIEVE 2.0 programs were used for protein analysis and label-free quantification. Heavy isotope labeled peptide EDQTSPAPGLR(13C6, 15N) was used as an internal standard for HR/SIM analysis of the samples from UIC.
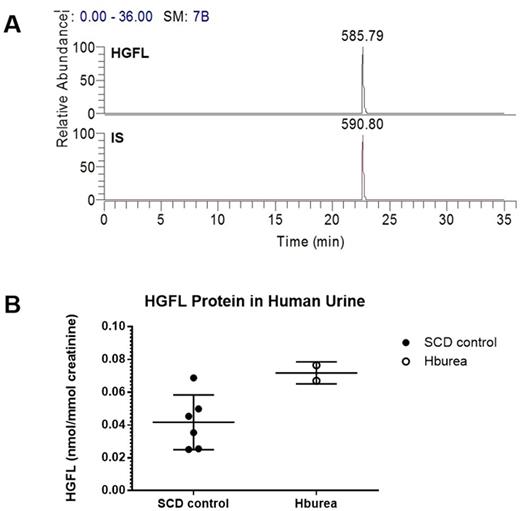
Results: Glomerular permeability assay was performed using six urinary samples collected from patients of the Center for Sickle Cell Disease, Howard University. Mass-spectrometry assay was performed for all samples. Samples with similar values of protein, albumin, creatinine, pH and SG, but different Palb activity were compared by SIEVE 2.0. Higher levels of hepatocyte growth factor-like (HGFL) protein were observed in three samples that induced glomerular permeability compared to three samples that did not. Since our attempts to produce antibodies to HGFL peptide for an ELISA assay were unsuccessful, we developed a HR/SIM method to measure HGFL peptide in urine. HR/SIM was performed for eight urine samples from the UIC cohort by measuring the ratio of ion peaks of HGFL peptide (m/z 585.79) and internal standard (IS) (m/z 590.80) (Fig. 1A). HGFL levels were found to be significantly increased (1.72-fold, p=0.015) in the urine samples of two patients at risk of developing renal disease based on the presence of hemoglobinuria compared to six samples without hemoglobinuria (Fig. 1B).
Conclusions: Combination of in vitro glomerular permeability assay with mass-spectrometry method of protein discovery and HR/SIM validation assay may be a useful platform for discovery of biomarker of early stage of CKD. Urinary level of HGFL may serve as a prognostic marker for development of CKD in SCD patients. A limitation of our study is the small number of samples used for validation.
Acknowledgments: This work was supported by NIH Research Grants 1P50HL118006, 1R01HL125005 and 5G12MD007597. The content is solely the responsibility of the authors and does not necessarily represent the official view of NHLBI, NIMHD or NIH.
HR/SIM analysis of HGFL peptide in human urine. (A)The ion peaks of HGFL peptide m/z 585.79 and internal standard (IS) m/z 590.80 were chosen for the high resolution/selective ion monitoring (HR/SIM) analysis using LTQ Orbitrap XL™ mass spectrometer. Extracted ion chromatograms (EICs) were based on a ±0.01 Da mass extraction window (MEW) centered on the theoretical m/z. (B) SCD patients at risk for developing renal disease based on the presence of hemoglobinuria showed significantly increased HGFL levels (1.72-fold, p=0.015).
HR/SIM analysis of HGFL peptide in human urine. (A)The ion peaks of HGFL peptide m/z 585.79 and internal standard (IS) m/z 590.80 were chosen for the high resolution/selective ion monitoring (HR/SIM) analysis using LTQ Orbitrap XL™ mass spectrometer. Extracted ion chromatograms (EICs) were based on a ±0.01 Da mass extraction window (MEW) centered on the theoretical m/z. (B) SCD patients at risk for developing renal disease based on the presence of hemoglobinuria showed significantly increased HGFL levels (1.72-fold, p=0.015).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.