Abstract
Background: Diagnosis of Gaucher disease (GD) can be difficult and diagnostic delays are common in both adult and paediatric patients. This may in part be attributable to the heterogeneous nature of early presenting signs and symptoms in GD, which may result in patients consulting various specialists before a diagnosis is reached. As part of this global Gaucher earlier diagnosis consensus (GED-C) initiative, a panel of expert physicians was asked to determine the most important barriers to diagnosis of GD. The panel was also asked about the impact of the initiative if its primary objective of facilitating earlier diagnosis was realized.
Methods: In round 1 of an anonymous, multi-stage, iterative Delphi process, the panel provided free-text answers to a series of open questions, including: "In your experience, what are the greatest barriers to diagnosis in patients with early GD?" and "Assuming that this initiative achieves its goal, what difference could it make to clinical practice?" An independent facilitator grouped the responses to these questions by theme, and these were checked and consolidated as summary statements by the two non-voting co-chairs of the GED-C initiative. In round 2, panel members independently rated the importance of each statement using a 5-point Likert scale (1 = not important, 5 = extremely important). Statements awarded an importance score of at least 3 by more than 75% of respondents were reissued in round 3, in which panel members rated their level of agreement with each statement using a 5-point pivoted Likert scale (1 = strongly disagree; 3 = neither agree nor disagree; 5 = strongly agree). Consensus was defined as more than 67% of respondents agreeing or strongly agreeing (a score of ≥ 4) with a statement.
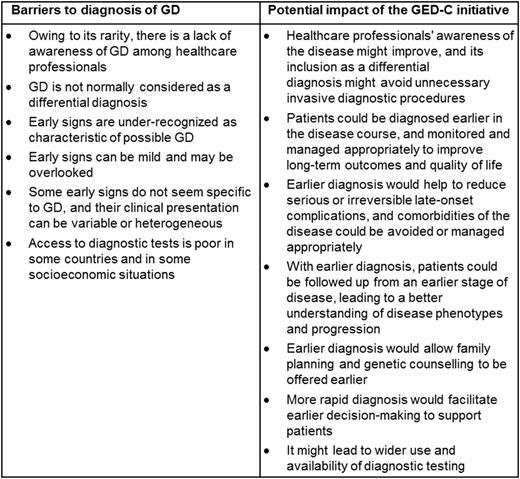
Results: In total, 22 experts from 16 countries were recruited to the GED-C panel. Round 1 (100% response, n = 22) yielded 47 phrases relating to barriers to diagnosis, and 30 relating to the impact of the initiative on clinical practice, which were consolidated as 9 statements describing barriers to diagnosis, and 8 summarizing the initiative's impact. In round 2 (100% response, n = 22) and round 3 (100% response, n = 22), 6 barrier statements and all 8 impact statements met the stipulated importance criteria, and consensus was then reached for all 6 barrier statements and for 7 impact statements (Table).
Discussion: This initiative highlights that, as well as the heterogeneous nature of GD, clinicians' lack of awareness of GD and poor knowledge of important presenting signs probably contribute to incorrect or delayed diagnoses. The GED-C initiative aims to resolve this by providing clinicians with simple guidance regarding important factors in GD, thereby improving disease awareness and facilitating early diagnosis. This may improve understanding of the natural history of GD, patient management, and potentially patients' long-term outcomes.
Acknowledgment: Submitted on behalf of the GED-C panel members and the European Hematology Association Scientific Working Group 'Quality of Life and Symptoms'. Administration of the GED-C initiative was funded by an unrestricted educational grant from Shire.
Kuter:Bristol-Myers Squibb: Research Funding; ONO: Consultancy; Protalex: Research Funding; Eisai: Consultancy; Shionogi: Consultancy; 3SBios: Consultancy; Syntimmune: Consultancy; MedImmune: Consultancy; CRICO: Other: Paid expert testimony; Amgen: Consultancy, Paid expert testimony; Pfizer: Consultancy; Rigel: Consultancy, Research Funding; GlaxoSmithKline: Consultancy; Shire: Consultancy; Genzyme: Consultancy. Salek:Novartis: Research Funding; Bristol-Myers Squibb: Research Funding; Shire: Consultancy; Servier: Consultancy; Sanofi: Research Funding; Agios: Consultancy. Mehta:Protalix/Pfizer: Honoraria, Other: travel grant, Research Funding; Genzyme: Honoraria, Other: travel grant, Research Funding; Actelion: Honoraria, Other: travel grant, Research Funding; Shire: Honoraria, Other: travel grant, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.