Abstract
Introduction: Bethesda assays, used in the quantification of factor VIII inhibitors, are time and labor intensive. They are performed by mixing one part of normal pooled plasma (NPP) with an equal amount of serial dilutions of patient plasma in imadizole buffered saline (IBS), and incubating at 37°C for 2 hours. After the incubation time, most laboratories perform factor VIII (FVIII) activities on each tube. These results are divided by the activity resulted on the control (NPP+IBS) to obtain the percent residual activity. The dilution with a percent residual FVIII activity between 25-75% is then converted to a Bethesda Unit using the Bethesda graph. This value is multiplied by the dilution to calculate final Bethesda units/mL.
We propose an alternative method, involving performing partial thromboplastin times (PTTs) on all dilution tubes and only performing FVIII activity testing on a limited number of tubes e.g. 2 tubes rather than all 10 tubes. The study below describes the methodology and provides data to show how the PTT can be used as a surrogate. Using PTTs may reduce cost per test by reducing the amount of FVIII deficient plasma used for FVIII activity testing, especially in the case of very high titer inhibitors.
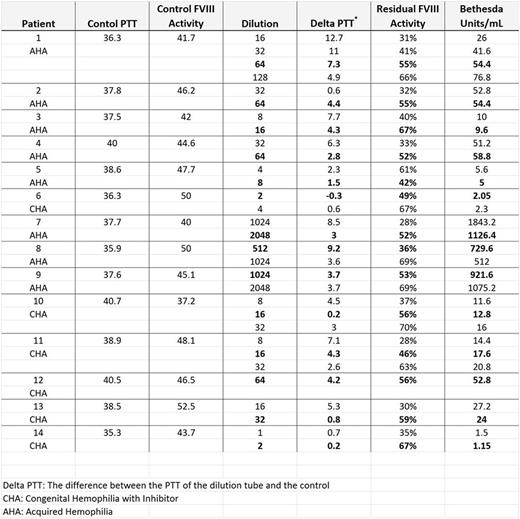
Methods: Serial dilutions of patient plasma with IBS are prepared to the 512 dilution (10 tubes) and incubated with an equal amount of NPP along with a control consisting of IBS and NPP. PTTs are run using Siemens Actin FSL reagent on the BCS coagulometer (Siemens) on all 10 tubes and the control. The tube with a resultant PTT within 5 seconds above the control is identified and then that tube, along with the next higher dilution, are tested for residual factor VIII activity (ACL TOP, Instrumentation Laboratory). These results are used to calculate the percent residual FVIII activity and Bethesda units/mL as described above. To support our hypothesis, we performed PTT and FVIII activities on all dilution tubes.
Results: We performed 14 inhibitor assays on 9 patients. 44% (4/9) had congenital hemophilia A with an inhibitor and 56% had acquired hemophilia A. Table 1 shows results from dilution tubes that resulted in residual FVIII activities between 25-75%. The dilutions with residual activity closest to 50% were used in the final interpretation and are bolded. Using the 5 seconds above control PTT to identify dilutions to limit FVIII assays correlated with residual FVIII activities in 12 of the 14 samples tested (86%). Furthermore, this method identified the dilution with the residual FVIII activities closest to 50% in 10 of the 12 samples.
Two samples demonstrated no correlation between PTT and FVIII activities. These samples had low titer inhibitors (<5 BU), which could have been predicted by the low PTTs in the first two dilutions. In these cases, serial FVIII activities were used in calculations. More data is needed to definitively say whether this methodology could not be used for low titer inhibitors.
Conclusion: An alternative method, using PTT testing to perform targeted FVIII activity testing, may be used in the Bethesda Assay.
Sarode:CSL Behring: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.