Abstract
Objective: Research on the efficacy and safety of non-factor drugs applied the etiological classification for treatment of hemophilia.
Diagnostic Methods: Between from June 2013 and June 2014, 73 patients were treated because of hemophilia. There were 69 males and 4 females, ages ranging from 0.4 to 83 years, with a mean of 16.13. We used MC-1000 Coagulometer (from MDC,Berlin, Germany) to detect the level of F¢ø/F¢ù:C, and detected the level of F¢ø/F¢ù inhibitor using Nigmegen assay. To detect mutations in the F8 and F9 gene, Intron 22 inversions were studied by inverse-shifting polymerase chain reaction (IS-PCR) and intron 1 inversions by long distance polymerase chain reaction. Point mutations were studied by sequencing the coding and promoter regions of the F8 and F9 genes.
Therapeutic Methods: Patients with hemophilia were prospectively treated with Non-factor drugs without replacement therapy of F¢ø/F¢ù factor. Patients with mutations in f8 and f9 genes were treated with orally Compound Hemophilia Capsule (Shaanxi Yida Hemophilia Institute, Pat. No:ZL201410130047X, Shaanxi Drug Approval No.Z20150044). Each capsules contain the traditional Chinese medicine raw materials as shown in the following: Ginseng Radix et Rhizoma 0.035g, Astragali Radix 0.117g, Angelica Sinensis Radix 0.035g, Paeoniae Radix Alba 0.07g, Ligustri Lucidi Fructus 0.058g, Ecliptae Herba 0.058g etc. specification: 0.3g/capsule*40 capsule/bollte. The effect of drugs was to enhance the activity and synthesis of coagulation factors in hepatocyte. The dose of 1.2g for adults administered three times daily from 3 months to 6 months. The LD50 of the drugs is 32g/Kg in the mice's acute toxicity test, which is equivalently to 533 times than the therapeutic dosage. In this study, no acute toxicity were observed. Other patients with normal f8 and f9 genes were treated with three kinds of drugs as shown in the following: Cyclophosphamide Injection (Shanxi Pude Pharmaceutical Co., Ltd, Shanxi province, China, H14023686). The dose of 0.2g for adults administered four-intravenous-drip daily from 4 weeks to 6 weeks. Compound Glycyrthizin Injection (Minophagen Pharmaceutical Co.,Ltd, Eisai China Inc. H20190124). The dose of 0.04g for adults administered four-intravenous-drip daily for 4 weeks. The effect of drugs was to stimulating the proliferation and protecting hepatocyte. Mycophemolate Mofetil Capsule (Cisen Pharmaceutical Co., Ltd. Shandong province. China, H20080764). The dose of 1.0g for adults administered twice daily by oral. The effect of drugs was immunosuppressive.
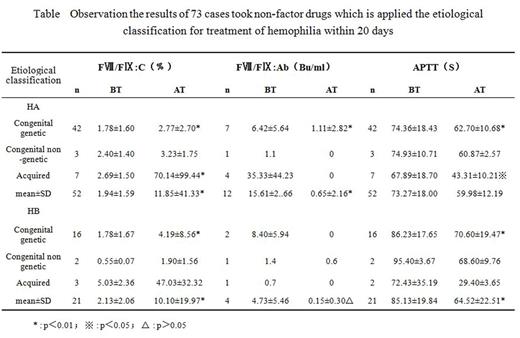
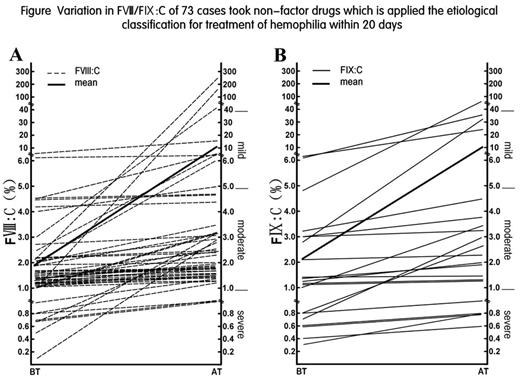
Results: Patients were classified by HA (52 cases, 71.23%, F¢ø:C in 1.94±1.59%) and HB (21 cases, 28.77%, F¢ù:C in 2.13±2.06%). There were 58 patients (79.45%) who were detected mutations with maternally inherited, 5 patients(6.85%) who detected mutations without maternally inherited, and 10 cases(13.70%) who had normal f8 and f9 genes. 20 days later after treatment, the level of F¢ø:C was raised from 1.94±1.59 to 11.85±41.33%(P£¼0.01), and the level of F¢ù:C was raised from 2.13±2.06% to 10.10±19.97%(P£¼0.01). However, the patients with normal f8 and f9 genes had the better curative effects (The results were shown in Table/ Figure). After one year, the level of F¢ø:C was 38.89±63.47% in 21 patients with HA, and the level of F¢ù:C was 17.60±32.16% in 10 patients with HB. None of patients reported adverse effects in clinical trials.
Conclusion: Non-factor drugs for treatment of hemophilia could promoted the level of F¢ø/F¢ù:C in varying degrees and reduced symptoms. Therefore, the study could provide a new insight into treatment of hemophilia. Further research was needed to understand the mechanism and curative effect.
The Institutional Ethics Committee approved the study (Approval No: 20120319).
Registration ID: WHO ICTRP & ChiCTR-OPC-16008561
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.