Abstract
INTRODUCTION:
Autologous stem cell transplant (ASCT) is the standard of care in transplant-eligible multiple myeloma (MM) patients and is associated witha significant improvement in progression-free survival (PFS), complete remission rates (CR), and overall survival (OS). However, the majority ofpatientsrelapse.
This study compares the efficacy of autologous hematopoietic stem cells followed by consolidation bybortezomibbased regimens to the no consolidation therapy in adult patients.
PATIENTS AND METHODS:
This is a retrospective study over a period of 7 years (2009-2015). All patients less than 65 years with a newly MM diagnosis were included. The protocol used in induction was VD (n=47) treatment whichconsisted of four 3-week cycles of 1.3 mg/m2 bortezomib administered subcutaneously (SC) on days 1, 4, 8, and 11 and 40 mg dexamethasone on days 1Ð4 and 9Ð12. Therapy with VTD was composed of four 3-week cycles of SC bortezomib and dexamethasone at the same doses and schedules as for the VD regimen plus 100 mg/day thalidomide administered orally. Therapy with VCD was composed of four 3-week cycles of SC bortezomib and dexamethasone at the same doses and schedules as for the VD regimen plus 500 mg/m2 cyclophosphamide administered orally on days 1, 8, and 15. Recommended concomitant medications included bisphosphonates, antibiotics, and antiviral prophylaxis. Acetyl salicylic acid was systematically used in the VTD arm. Stem cells were mobilized with 15 or 10 microg/kg G-CSF alone. Leukapheresis to harvest stem cells was performed on day -2 and -1. The grafts were kept in a conventional blood bank refrigerator at 4¡C until reinfusion on day 0. The target yield was 2 x106 CD34+ cells/kg. Following induction therapy, all patients had to proceed to ASCT. The conditioning regimen consisted of melphalan 200 mg/m2 in all patients.The consolidation regimen consisted of two cycles of VD or VCD or VTD after autologous stem- cell transplantation. In our study patients were divided into two groups: Group1 (ASCT plus consolidation) and Group 2 (ASCT alone).
The therapeutic evaluation focused on the overall response (CR + VGPR) and progression free survival (PFS) and overall survival (OS) calculated by the Kaplan-Meier method.
RESULTS:
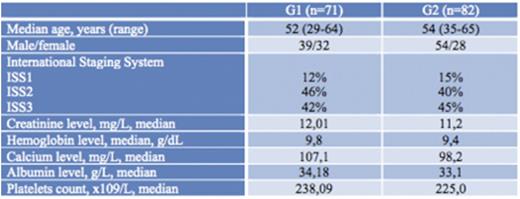
Over a period of 7 years, 153 patients were collected divided in two group: G1 (n=71) and G2 (n=82). Baseline characteristics are summarized in Table 1. No significant difference was observed between the 2 groups.
In terms of CR, 58% of the patients in the G1 achieved a CR after consolidation vs 33% in the G2 after ASCT alone (p=0.007). In terms of VGPR, 31% of the patients in the G1 achieved a least a VGPR vs 17% in the G2 (p=0.04). The relapse rate was significantly lower in the G1 (10%) than the G2 (39%), (p=0.0001).
The median follow-up period was 23,4 months. PFS was significantly higher in the G1, median no reached vs 37 months in the G2 (p=0.02) but no significant difference was observed in terms of OS rate between the 2 groups, 91% (G1) versus 82% (G2) at 27 months (p=0.7).
CONCLUSION:
We conclude thatbortezomib-based regimens as consolidation therapy after ASCT in patients with MM was effective in the improvement of PFS and response rate.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.