Abstract
5-Azacytidine is a cytosine analog and a potent DNA methyltransferase inhibitor, previously shown to induce DNA demethylation. 5-Azacytidine is indicated for the treatment of adult patients with intermediate-2 and high-risk myelodysplastic syndrome (MDS) and acute myeloid leukaemia (AML) with <30 % blasts or of patients aged ≥65 years with AML who are not eligible for HSCT. NK cells are an important component of immunological tumor surveillance and their role in MDS pathogenesis is of rising interest. The role of immunosuppressive myeloid derived suppressor cells (MDSCs) in NK cell cytotoxicity and their correlation with lymphocyte subpopulations, is currently under investigation. With regard to MDS, increased NK-cell mediated cytotoxicity was found in one study, while several other studies reported impaired NK cell function ( Chamuleau ME et al. Hematologica 2009;94(4):496-50, Kiladjian JJ et al. Leukemia 2006;20(3):463-470, Epling-Burnette PK et al., Blood 2007;109(11):4816-482.
We investigated 5-Azacytidine's impact on human in vitro NK cell cytotoxicity and in parallel its impact on frequencies of peripheral blood T, NKT, NK, Tregs and MDSCs cell subpopulations.
To elucidate the immunological effects of 5-Azacytidine , we collected blood samples from 17 AML/MDS patients (age: 40-86y, median: 76y) and 9 healthy donors (age: 34-58y, median: 54y). Informed consent was obtained from all patients and donors according to the Declaration of Helsinki. Eleven patients suffered from MDS-RAEB II, three from secondary AML (MDS related) and three from AML. All patients had received at least 3 5-Azacytidine cycles (median 10, ranged from 3 -32). Complete remission was achieved by 47% of patients.
Peripheral blood mononuclear cells (PBMC) were isolated from patients and healthy donors using density gradient centrifugation.
CD56+/CD3- NK cells were purified from PBMCs by negative immunomagnetic selection. Purified NK cells were tested against the NK resistant Raji cell line and the NK sensitive K562 cell line, at a ratio of effector to target cells 5:1 . Cytotoxicity was measured in duplicate samples using a 4-h cytotoxicity assay at 37°C. Target cells were labeled with PKH-67stain and the analysis of cell viability was determined by staining with 7-AAD and was restricted to the PKH-67+ fraction. The mean proportion of 7AAD positive cells from the duplicate samples was determined. Background target cell death was determined from cells incubated in the absence of effector cells. Cell-mediated cytotoxicity was reported as the percentage of killing over background cell death averaged from the two samples.
Frequencies of T, NKT, NK, Tregs and MDSCs cell populations were measured by flow cytopetry the same day as the cytotoxicity test was performed. MDSCs were phenotypically defined as CD33+/CD11b+/CD14-/HLA-DR lo/- .
Statistical significance was determined by two-tailed unpaired t-test. Grouped data were expressed as mean ± standard error of the mean.
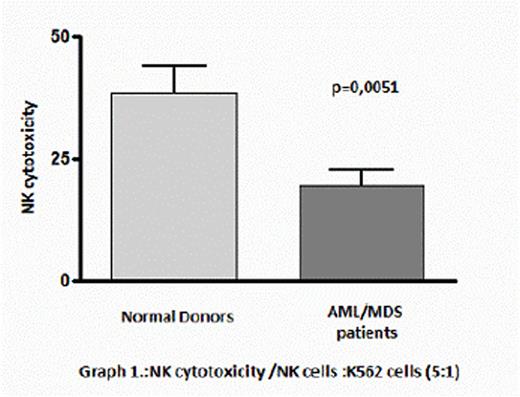
The in vitro cytolytic capacity of AML/MDS-NK cells was investigated first against the erythroleukemia cell line K562, which represents a highly susceptible NK cell target and was presented significantly decreased compared with healthy donors (19,3±3,33 vs 38,3±5,68, p=0,0051)(graph 1.).
In vitro cytotoxicity of AML/MDS-NK cells against NK resistant Raji cell line didn't show any difference between the two studied cohorts (patients:1,93±0,44 vs healthy donors:3,41±0,83, p=0,1).
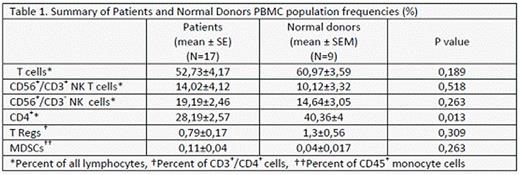
Overall frequencies of T, NKT, NK, TRegs and MDSCs cells were comparable among AML/MDS patients and healthy controls as it is presented in table 1, while CD3+/CD4+ subpopulation showed significant reduction in the patient's cohort.
Natural Killer cell, well known to mediate anti-leukemic responses, showed defective in vitro cytoxicity in patient's cohort on 5'Azacytidine treatment , implying further in vivo impaired immune surveillance. Notably no reduced NK levels were found, nor any alterations in frequencies of MDSCs and Tregs were noted in patient's cohort.
As it is already presented, MDS patients show a significant increase in MDSCs that negatively correlates with lymphocyte populations (Michelle K. Gleason et al.Blood, 2014; 123 (19):3016-3026).
5-Azacytidine treatment may confer to restitution of this impairment, conferring to meliorate antitumor immunity.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.