Abstract
Introduction: Bendamustine hydrochloride (bendamustine; Treanda®) is an alkylating agent approved globally for multiple indications including chronic lymphocytic leukemia, indolent B-cell NHL, mantel cell lymphoma, and multiple myeloma. The plasma concentration-time profile of a single intravenous (IV) dose of bendamustine follows a triphasic pattern, with a very rapid distribution and intermediate phases followed by a slow terminal decline. The initial phases represent most of the observed area under the plasma drug concentration-by-time curve (AUC), and the effective half-life (t1/2) of 40 minutes is based on the elimination rate of the intermediate phase. Two active circulating metabolites (gamma-OH-bendamustine [M3] and N-desmethyl-bendamustine [M4]) are produced via the CYP1A2 pathway, but both metabolites are generally found at low levels relative to parent bendamustine. However, relatively little is known regarding the effect of race/ethnicity on the pharmacokinetics (PK) of bendamustine. The present investigation had the goal of defining the PK of bendamustine in a subset of ethnic Chinese patients with relapsed/refractory iNHL from an open-label, single-arm, multicenter study in China.
Methods: Patients with documented relapsed indolent B-cell NHL after rituximab treatment and 1-3 previous chemotherapy regimens were included. Patients received bendamustine IV 120 mg/m2 infusion (over 60 minutes and not more than 120 minutes) on day 1 and 2 of each 21-day cycle for at least 6 cycles and were allowed to continue treatment for up to 8 cycles. Blood samples for PK were collected at the following time points on day 1 of cycle 1: before, during, and immediately after the infusion, and 15, 30, and 45 min, and 1, 2, 4, 6, 8, 10, 12, and 24 h after the end of infusion. PK parameters were estimated using noncompartmental methods. The PK evaluable population included those patients for whom at least one PK parameter was calculable.
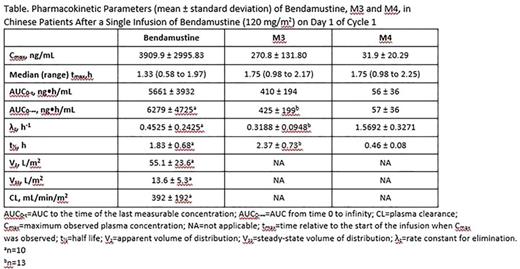
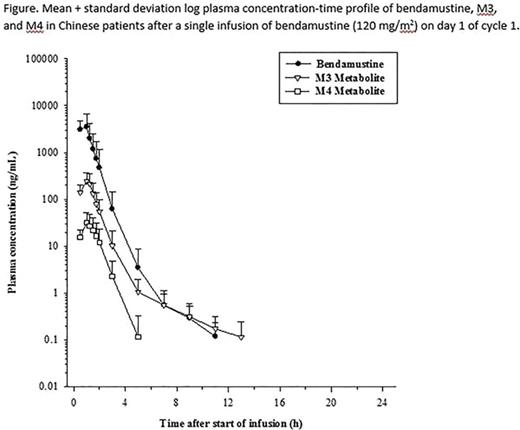
Results: PK parameters were characterized in 15 Chinese patients with relapsed/refractory iNHL. Results of the analyses are summarized and presented in the table. Highest plasma concentrations were observed at either the midpoint or end of infusion in all patients. The subsequent decrease in plasma concentration occurred in a multiphasic manner as shown in the figure. The data suggested that bendamustine has a high plasma clearance and does not readily distribute beyond the extracellular space. The metabolites M3 and M4 were observed at lower concentrations than parent bendamustine. Total systemic exposure (AUC0-∞) to M3 was approximately 1/10 of that to bendamustine and approximately 10-fold higher than that to M4.
Conclusion: The PK characteristics of bendamustine and its known active circulating metabolites, M3 and M4, in Chinese patients with cancer are generally consistent with those reported in other ethnic populations.
Sponsor: Teva Branded Pharmaceutical Products R&D
Hellriegel:Teva Pharmaceuticals, Inc.: Employment, Equity Ownership. Robertson:Teva Pharmaceuticals, Inc.: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.