Abstract
Introduction: Molecular alterations involved in development of classical Hodgkin lymphoma (cHL) are only partially known. Genetic alterations in NFkB pathway and the imbalance of T regulatory (Treg) and TH17 lymphocytes has been recognized as critical pathogenetic mechanism involved in immune scape and blockade of apoptosis in Reed-Sternberg cells. Emerging evidences suggests that NFkB activation can promote lymphocyte proliferation and survival, and therapeutic inhibition of the NFkB pathway have been proposed. Recently, our group showed an increase of circulating Treg cells in patients with cHL at diagnosis, however, after therapy, elevated levels of circulating TH17 cells was observed. Increased frequencies of Treg lymphocytes in the tumor microenvironment and peripheral blood have been proposed as one of the mechanisms for this anergic state. TH17 exhibit effector functions in immune system and tumor-infiltrating TH17 cells are associated with better prognosis in human.
Objectives: In this study we aimed to evaluate the immune gene expression profile in whole blood of cHL patients at diagnosis and after treatment, and evaluate pathways and gene interaction networks.
Methods: This is an open multicenter study and, so far, we included 51 patients consecutively from February 2011 to November 2015. Twenty consecutively diagnosed cHL patients, with whole blood RNA extracted at diagnosis and after treatment, were recruited for this study and prospectively evaluated. All patients were HIV negative and received ABVD chemotherapy protocol and radiotherapy if necessary. The general expression of 96 messengers RNAs present in the peripheral blood and involved in immune response was performed by a customized quantitative real-time PCR array (TaqMan¨ Low Density Array). The data was normalized with B2M mRNAs levels and relative gene expression was calculated by the 2^DDCt method, considering Wilcoxon test and Benjamini-Hochberg adjustment to correct p-values. The differentially expressed genes were used to conduct functional/pathway enrichment analysis and construct gene interaction networks. Functional annotation networks were generated using Ingenuity Pathway Analysis (IPA; Ingenuity Systems) software.
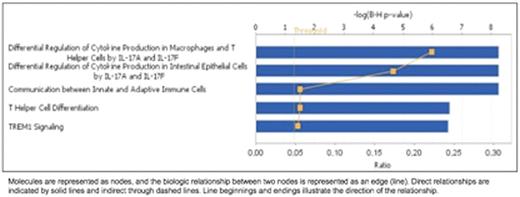
Results: From the 20 patients included for this study, 12 (60%) were male, 5 (31%) had Epstein Barr virus related cHL, 18 (90%) patients presented with B symptoms, 19 (95%) patients had advanced diseases at diagnosis (stage IIBX, III and IV) and 10% of patients relapsed. We considered paired samples from 15 patients before and after treatment. At diagnosis we observed that cHL patients presented higher expression of CD274 (2 fold, p=0.018), CD28 (1.5 fold, p=0.041), CTLA-4(1.5 fold, p=0.004), FAS (1.4 fold, p=0.041), ICOS (2.1 fold, p=0.015) and IL-10 (2.7 fold, p=0.002), and decreased expression of CCL2 (-2.7 fold, p=0.026), CCL5 (-1.6 fold, p=0.012), CD40 (-1.9 fold, p=0.003), CSF2 (-1.9 fold, p=0.012). We found no association between clinical and epidemiological characteristics with immune gene expression profile. We have found that after treatment, patients displayed a specific gene expression profile related to the top 5 canonical pathways summarized in following figure:
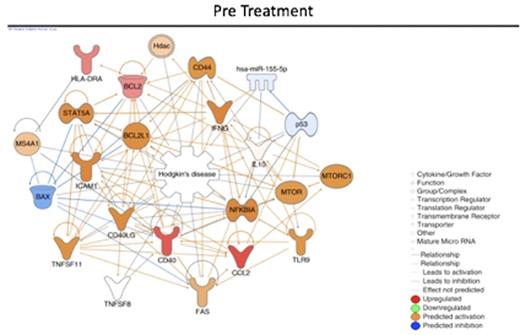
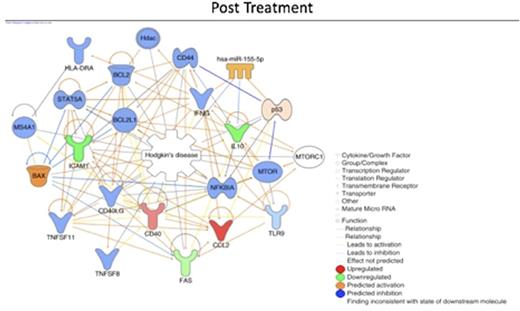
We also built a biological network to investigate the connection between the differentially expressed genes and to predict the status (activation/inhibition) of the other connected genes (nodes). The in silico analysis revealed that NFkB, a node which its signaling activation is predicted to be activated before treatment and inhibited after (following figures). The network also showed different central node molecules (molecules connected with multiple other nodes from the network), that are indirectly related to Hodgkin lymphoma and should be further investigated as potential drugable targets.
Conclusions: In this study, we showed that, at diagnosis, cHL patients presented an inflammatory gene expression profile in blood that changes after treatment to an effector/immunological one. NFkB is predicted to be activated before treatment and inhibited after ABVD chemotherapy and radiotherapy. This kind of system biology approach helped us to understand that cHL associated immunosuppression and the immune reconstitution after treatment maybe the key to develop new prognostic factors and treatment strategies as well as identify drugable targets.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.