Abstract
BACKGROUND: Diffuse large B-cell lymphoma, not otherwise specified (DLBCL), though defined as one disease entity, consists of two main cell-of-origin (COO) subtypes, germinal center B-cell like (GCB) and activated B-cell like (ABC), with the latter predicting a significantly worse prognosis using conventional chemotherapy. Up to 40% of all patients diagnosed with DLBCL will experience relapse, and outcomes are generally poor in this setting. In this study, we examine the proportion of GCB vs. non-GCB subtypes at initial diagnosis and at time of relapse, and assess the incidence of reclassification from one subtype to another.
METHODS: Patients were prospectively enrolled in the University of Iowa/Mayo Clinic SPORE Molecular Epidemiology Resource (MER) within 9 months of diagnosis and followed for relapse, retreatment, and death. All relapse events were validated by review of the medical record. This analysis includes patients diagnosed with DLBCL who underwent initial treatment and subsequently experienced relapse as confirmed by biopsy. Immunohistochemical staining data including CD10, Bcl-6, and Mum-1, were collected from available pathology reports and classified to either GCB or non-GCB subtypes as predicted by the Hans algorithm. We compared subtype classification and individual immunohistochemical data both at the time of diagnosis and relapse. The overall subtype agreement between the two diagnostic time points was assessed.
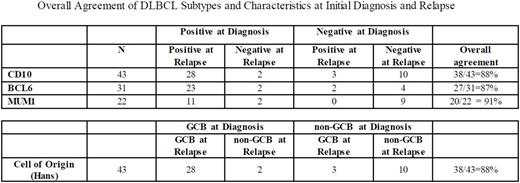
RESULTS: 1023 patients with newly diagnosed DLBCL were enrolled in the MER from 2002 - 2012. Of those, 249 had documented disease relapse. Of those analyzed, a total of 43 patients had COO classification based on the Hans algorithm from tissue biopsy at both diagnosis and relapse. Thirty patients were characterized as GCB DLBCL on initial presentation, and upon relapse 28 were noted to be GCB, while 2 were noted to be the non-GCB subtype. Of the 13 that were characterized as non-GCB on initial presentation, 10 remained non-GCB while 3 were reclassified as GCB. The overall agreement of DLBCL COO phenotypes between initial diagnosis and relapse was 88%. Seven percent of those initially diagnosed as GCB were reclassified as non-GCB subtype; in contrast, 23% of those initially diagnosed as non-GCB had changed to GCB upon relapse. Similar analysis was performed on individual immunohistochemical staining factors including CD10, Bcl-6, and Mum-1, and we observed overall agreement of 88%, 87%, and 91%, respectively.
CONCLUSIONS: A majority of patients with relapsed DLBCL exhibit similar COO phenotypes at initial presentation and at relapse, and the incidence of reclassification from one subtype to another is uncommon. This suggests that the initial treatment regimen for DLBCL rarely alters the basic cancer phenotype when immunohistochemistry laboratory technical factors and pathologist interpretive discrepancies are excluded as causes for this change. However, reclassification from GCB to non-GCB subtypes is observed in a minority of cases, which may affect the overall outcome and response to treatment. Further studies are needed using gene expression to categorize COO and to examine the factors surrounding the time of relapse for possible harbingers of relapse as well as the behavior and outcome of reclassified DLBCL.
Ansell:BMS, Seattle Genetics, Merck, Celldex and Affimed: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.