Abstract
Background
Patients with multiple myeloma (MM) have a 9-fold increased risk of developing venous thromboembolism (VTE). Current guidelines recommend pharmacologic thromboprophylaxis in patients with MM receiving an immunomodulatory agent in the presence of additional VTE risk factors (NCCN 2015, ASCO 2014, ACCP 2012). However, putative risk factors vary across guidelines and no validated VTE risk tool exists for MM. Khorana et al. developed a VTE risk score in patients with solid organ malignancies and lymphoma (Blood, 2008). We sought to apply the Khorana et al. score in a population with MM.
Methods
We identified patients diagnosed with MM within the Veterans Health Administration (VHA) between September 1, 1999 and December 31, 2009 using the International Classification of Diseases (ICD)-03 code 9732/3. We followed the cohort through October 2014. To eliminate patients with monoclonal gammopathy of undetermined significance and smoldering myeloma, we excluded patients who did not receive MM-directed therapy within 6 months of diagnosis. We also excluded patients who did not have data for hemoglobin (HGB), platelet (PLT) count, white blood count (WBC), height and weight, as these are all variables included in the Khorana et al. risk model. Height and weight were assessed within one month of diagnosis and used to calculate body mass index (BMI). We measured HGB, PLT count, and WBC count prior to treatment initiation: within two months of MM diagnosis. A previously validated algorithm, using a combination of ICD-9 code for VTE plus pharmacologic treatment for VTE or IVC filter placement, identified patients with incident VTE after MM diagnosis (Thromb Res, 2015). The study was approved by the Saint Louis VHA Medical Center and Washington University School of Medicine institutional review boards.
We calculated VTE risk using the Khorana et al. score: We assigned 1 point each for: PLT ≥ 350,000/μl, HGB < 10 g/dl, WBC > 11,000/μl, and BMI ≥ 35 kg/m2. Patients with 0 points were at low-risk, 1-2 points were considered intermediate-risk and ≥3 points were termed high-risk for VTE.
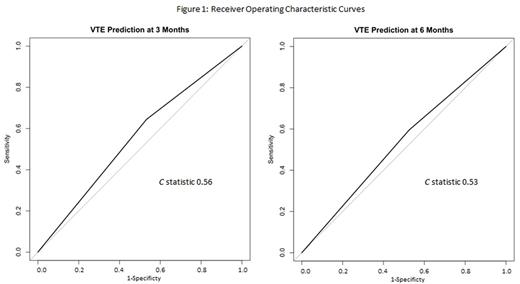
We assessed the relationship between risk-group and development of VTE using logistic regression at 3- and 6-months. We tested model discrimination using the area under the receiver operating characteristic curve (concordance statistic, c) with a c-statistic range of 0.5 (no discriminative ability) to 1.0 (perfect discriminative ability).
Results
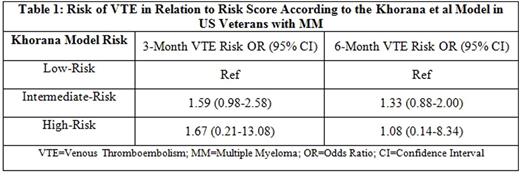
We identified 1,520 patients with MM: 16 were high-risk, 802 intermediate-risk, and 702 low-risk for VTE using the scoring system in the Khorana et al. score. At 3-months of follow-up, a total of 76 patients developed VTE: 27 in the low-risk group, 48 in the intermediate-risk group, and 1 in the high-risk group. At 6-months of follow-up there were 103 incident VTEs: 41 in the low-risk group, 61 in the intermediate-risk group, and 1 in the high-risk group. There was no significant difference between risk of VTE in the high- or intermediate-risk groups versus the low-risk group (Table 1). The c-statistic was 0.56 at 3-months and 0.53 at 6-months (Figure 1).
Conclusion
Previously, the Khorana score was developed and validated to predict VTE in patients with solid tumors. It was not a strong predictor of VTE risk in MM. There is a need for development of a risk prediction model in patients with MM.
Carson:American Cancer Society: Research Funding. Gage:National Heart, Lung and Blood Institute: Research Funding. Kuderer:Janssen Scientific Affairs, LLC: Consultancy, Honoraria. Sanfilippo:National Heart, Lung and Blood Institute: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.