Abstract
Background: Previously, real-world baseline characteristics and response monitoring pattern of Chinese CML patients treated with dasatinib were poorly studied and rarely reported. The Chinese dasatinib registry CA180-518 is a multicenter, observational study that provides us with a unique opportunity to obtain such information. Here we reported baseline characteristics and monitoring adherence of patients enrolled in this study.
Methods: Imatinib-resistant/-intolerant CML adult patients in any phase, who planned to receive or were receiving dasatinib therapy based on physician's clinical judgement, were enrolled in this study and were separated into 3 cohorts based on their disease phases: chronic phase (CP), accelerated phase (AP) and blast phase (BP). Patients were to be followed until death, withdrawal of consent, end of study or loss of follow-up. Patient visit schedule, evaluation and treatment decision were determined solely by the physician in their real-world clinical practice. The first and the second patient visits were designed at Month 0 and Month 3.
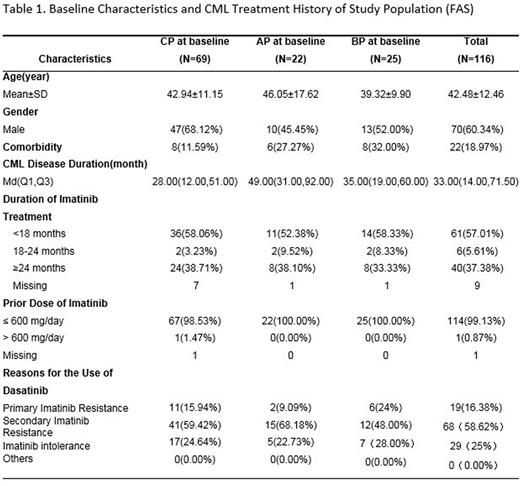
Results: From April 2013 to June 2015, a total number of 126 patients from hematology division of 28 hospitals in China were enrolled. Of these, 116 were included in the full analysis set (FAS), including 69 patients with CML-CP, 22 patients with CML-AP and 25 patients with CML-BP. Patients in the FAS had a median age of 43 years. 60.34% (70/116) were male. ECOG performance status (PS) at diagnosis was available for 81.90% (21/116) patients and most of them (75.00%, 87/116) had a good PS (PS0-PS1). Median disease duration was 33.00 months (Q1-Q3:14.00-71.50). 37.38% (40/116) of the patients had received imatinib treatment for ≥ 24 months. 75% (87/116) of the patients used dasatinib due to primary or secondary imatinib resistance and 25% (29/116) due to imatinib intolerance. 69 of 116 patients had undergone genetic mutation test at baseline, among which, 76.80% (53/69) were detected with genetic mutations. Y253F/H (15.94%; 11/69), E255K/V (11.59%; 8/69) and F359V/I (10.14%; 7/69) were the three most common mutation types, which also happened to be the mutations associated with nilotinib-resistance.
18.97% (22/116) patients had baseline comorbidities and 50% (11/22) of them had ≥2 comorbidities. The most common comorbidity was cardiovascular diseases (CVD)/metabolic syndrome (MS) (54.54%, 12/22). It is also important to note that 22.72% (5/22) patients had a history of hepatitis B virus (HBV) infection.
For 62 CML-CP patients who completed the second visit, 64.52% (40/62) had taken routine blood test, 72.58% (45/62) had taken PCR test and 22.58% (14/62) had taken cytogenetic test. It is noteworthy that there were still 19.35% (12/62) patients who took none of the laboratory tests mentioned above. The efficacy of dasatinib is being assessed based on the test results and will be reported in the further.
Conclusion: The results from this analysis provided real-world data about baseline characteristics and response monitoring pattern of Chinese CML patients treated with dasatinib. Although less than 20% patients had baseline comorbidities, the fact that the most common comorbidity was CVD/MS should raise awareness, because TKI-related vascular AEs (VAEs) may develop preferentially in patients with these preexisting risk factors. Although the frequency of VAEs is lower in patients receiving dasatinib compared with nilotinib or ponatinib, for these patients, it is still important to closely monitor metabolic and cardiovascular parameters during the follow-up to reduce the vascular risk. Meanwhile, for patients who are carriers of HBV, EMA recently recommended that signs and symptoms of active HBV infection should be closely monitored throughout therapy and for several months following termination of therapy, given the risk of HBV reactivation for all TKIs.
Besides, patient adherence to monitoring is a concerning problem for CML management in China. This data indicates a considerable amount of CML patients, who do not follow current recommended guidelines on response monitoring.
Acknowledgment: BMS funded this research and medical writing support
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.