Abstract
Introduction Imatinib mesylate (Glivec, Novartis) is the first tyrosine kinase inhibitor (TKI) targeting the BCR-ABL1 fusion protein responsible for the pathogenesis of chronic myeloid leukemia (CML). Low cost generic alternatives to imatinib are an integral part of cost effective healthcare strategies for developing countries. However, the use of generics has been associated with different clinical outcomes. In this study, we compared outcomes of two groups of patients who received Glivec as first-line therapy (Group 1) to patients who received generic imatinib as first-line therapy (Group 2) in Bosnia and Herzegovina.
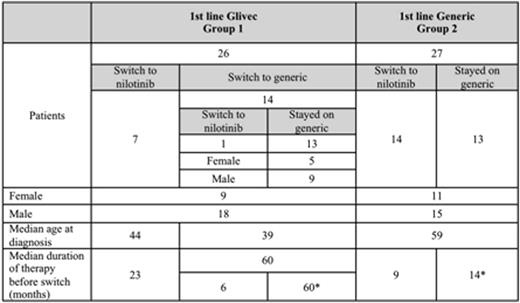
Material and methods This was a multicenter retrospective cohort study of BCR-ABL1 positive CML patients (n = 53) in the Federation of Bosnia and Herzegovina between 1 June 2005 and 31 March 2016. Glivec was used from 01 June 2005 until 30 September 2013, when all patients had to switch to generics, which was mandated by the Federal Solidarity Fund that allocates targeted cancer therapies. The following generic imatinib was available: Anzovip (Zdravlje, Actavis) from 09/2013 to 09/2014, Meaxin (Krka) from 09/2014 to 12/2015, and Plivatinib (Pliva) from 12/2015. Patient data was collected from the database of the Federal Solidarity Fund, a subsidiary of the Federal Health Insurance Agency. Branded and generic imatinib was administered orally at dosage of 400 mg/day. Patients who were switched to nilotinib received orally 400 mg/day. Patients on Glivec included in this study started therapy from 0-6 months from time of diagnosis, while patients who started with generics did not wait for therapy. Patient variables that were collected included age, gender, town, date of diagnosis, date of start of therapy, monthly TKI dosage, adverse side effects, progression, lethal outcome, prognostic factors and diagnostic parameters, including cytogenetics and molecular testing. In September 2013, Glivec stopped being available in Bosnia and all CML patients were switched to generic therapy Anzovip. Median duration of each therapy is given in Table 1.
Results We compared patients on Glivec as first-line therapy (Group 1, n=26) to patients on first-line generic imatinib (Group 2, n=27) with the follow-up period of at least three years for each group. When we compare Groups 1 and 2 using intention to treat analysis, Kaplan-Meier estimated rate of overall survival at 24 months of therapy was 88% vs. 68%, respectively (p=0.14), while 69% vs. 70% achieved CCyR (p=0.12), respectively. In Group 1, 27% (7/26) patients switched to nilotinib (treatment failure in 2 patients and side effects in 5 patients), 54% (14/26) patients switched to generics because Glivec was no longer available, and 19% (5/26) patients stopped therapy (2 patients stopped therapy and 3 patients died). Of the 7 patients who switched to nilotinib, 71% (5/7) achieved CCyR, 29% (2/7) achieved MMR and none died. Of 19 patients who stayed on imatinib, 68% (13/19) achieved CCyR, 63% (12/19) achieved MMR and 3/19 (16%) died. Of the 54% (14/26) patients who were switched from branded imatinib to generic imatinib, one patient (7%) lost complete cytogenetic response.
Regarding Group 2, 52% (14/27) of patients switched to nilotinib due to treatment failure (n=8) and side effects (n=6), while 48% (13/27) of patients stayed on generics. Of patients who switched to nilotinib, 43% (6/14) achieved CCyR and 15% (2/14) achieved MMR. Of the patients who stayed on generic imatinib, 100% (13/13) achieved CCyR and 85% (11/13) achieved MMR.
Conclusion Our results suggest that there was no obvious difference in the treatment efficacy between generic and branded imatinib. At 3 years, there was no significant difference in the overall suvival and achievement of CCyR between first-line Glivec and first-line generic imatinib (p=0.14, and p=0.12, respectively).
* Median duration of therapy on generic imatinib
Radich:Bristol-MyersSquibb: Consultancy; Pfizer: Consultancy; ARIAD: Consultancy; TwinStrand: Consultancy; Novartis: Consultancy, Other: laboratory contract.
Author notes
Asterisk with author names denotes non-ASH members.