Abstract
INTRODUCTION: Second and third generation tyrosine kinase inhibitors (TKIs) are available for Philadelphia positive chronic myeloid leukemia (Ph+ CML) treatment, but though median age at diagnosis is 60-65 years, elderly patients are under-represented in clinical trials concerning 2G-TKIs and a very small number of papers has been published with series of CML patients > 65 years treated with 2G-TKIs or ponatinib.
AIM: we have reviewed our single-center experience with 2G-TKIs in chronic-phase CML (CP-CML) patients > 65 years, in first or subsequent therapeutic lines.
PATIENTS AND METHODS: the patients were 10 females and 13 males; CML diagnosis had been made between 1994 and 2015 at a median age of 67 years (61-77). According to Sokal score, 3 patients (13%) were classified as low, 15 (65%) as intermediate and 5 (22%) as high-risk respectively. B3a2 transcript was detected in 13 (56%), b2a2 in 10 (44%) cases.
Overall, 30 therapies with 2G-TKIs were prescribed in 23 patients CP-CML > 65 years old, from 2007 to 2015. At 2G-TKI therapy start the median age was 68 years (65-79). Charlson Comorbidity Index (CCI) score was 0 in 11 patients, 1 in 4 patients, 2 in 4 patients, 3 in 3 patients and 4 in 1 patient.
Thirteen of 23 patients received second-generation TKIs (2G-TKIs) up-front (6 nilotinib, 7 dasatinib), 10/23 patients as 2nd line therapy (6 nilotinib, 4 dasatinib) after imatinib failure (8/10) or intolerance (2/10), median time from imatinib start to 2G-TKIs being 18 months (9-120). Five of 23 patients needed a TKI as 3rd therapeutic line (4 dasatinib, 1 bosutinib), 1 patient also as 4th (ponatinib) and 5th line (bosutinib).
RESULTS: all 23 patients are alive after a median follow up of 45 months (12-259) from diagnosis; median follow up from the first 2G-TKI treatment is 36 months (12-110), in details 37 months (12-110) for patients that received 2G-TKIs up front and 34 months (12-70) for patients that received first 2G-TKI as second line therapy.
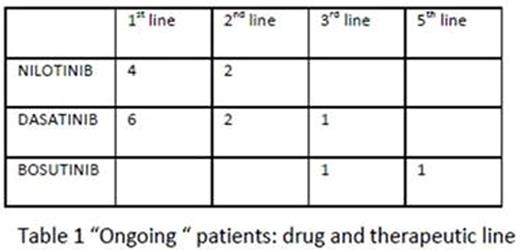
Seventeen of 23 patients (74%) are still on 2G-TKI therapy, with a median treatment of 37 months (12-110) -table 1-. At the last visit 15/17 patients had MMR or best results (1 MR4.5, 9 MR4, 4 MR3).
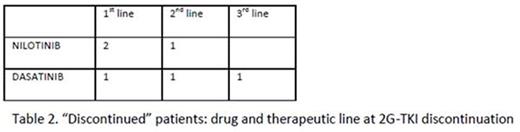
Six patients of 23 (26 %) stopped definitively 2G-TKI therapy, after a median treatment duration of 31 months (6-68) from the first 2G-TKI (table 2), in all cases for non-hematologic toxicity: 1 recurrent pleural effusion (grade 2), 1 geriatric syndrome, 1 carotid atherosclerotic disease, 1 PAOD, 1 vomiting (grade 3) and 1 pulmonary hypertension. At discontinuation, the disease response was MMR (2 patients), MR4 (3 patients), MR 4.5 (1 patient).
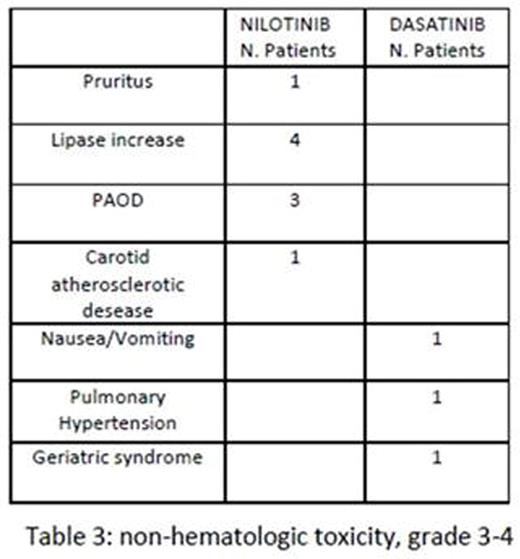
Hematologic toxicity occurred during 2G-TKI treatment in 7/23 patients (30%), grade 1-2 in 4, grade 3-4 in 3. Sixteen of 23 (69%) patients experienced non-hematologic toxicity, grade 1-2 in 7 (30%) -5/7 pleural effusion-, and grade 3-4 in 9 (39%) -table 3-.
There were no significant differences between patients still on therapy and patients that completely discontinued 2G-TKIs regarding Sokal score risk and b2a2/b3a2 transcripts. Conversely, CCI differed in these 2 categories, because CCI was > 1 in 5/6 (83%) of "discontinued" patients and in 7/17 (41%) of "ongoing" patients. Among the eleven patients with CCI = 0, only 1 discontinued 2G-TKI treatment.
CONCLUSIONS: Our experience shows that 2G-TKIs are very effective in elderly CP-CML, not only as first line therapy. Non-hematologic toxicity is high and it is the main reason for 2G-TKI discontinuation, that appears to be related to comorbidities. The vast majority of patients without comorbidities remain on 2G-TKI treatment long term.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.