Abstract
Introduction:
High risk myelodysplastic syndromes (MDS) and acute myelogenous leukemia (AML) commonly affect individuals of advanced age with multiple co-morbidities unsuited for curative standard treatments with allogeneic stem cell transplantation (allo-SCT) or intensive chemotherapy (ICT). New reduced intensity therapeutic approaches bringing improved life expectancy are needed for such patients. We conducted a phase I clinical trial to evaluate the safety and activity of sequential therapy with low-dose clofarabine followed by lenalidomide in older high risk MDS or AML. We hypothesized that lympho-depletion by clofarabine could "reboot" lymphocyte reactivity against the malignancy and promote favorable immune modulation by lenalidomide. Here we report preliminary clinical outcomes and associated cellular and molecular immune profiles.
Methods:
Four subjects (median age 68 years, range-60-79) with relapsed or refractory high-risk MDS (IPSS risk score>intermediate 2) or AML were enrolled to the protocol (12-H-0146, clinicaltrials.gov ID: NCT01629082). All subjects had been previously treated with at least one hypomethylating agent. Subjects received a single course of intravenous low-dose clofarabine 5mg/m2/d for five days, followed by consolidation therapy with oral lenalidomide with dose escalation from 25 mg daily up to 50 mg daily for 2 cycles. In the absence of dose limiting toxicity (DLT) or disease progression, subjects received lenalidomide maintenance 10 mg daily in 28 day cycles, with dose adjustments, for up to 12 cycles. Blood and marrow samples were collected before starting clofarabine, before consolidation with lenalidomide, and during maintenance. Multiparameter flow cytometric analysis was performed to characterize T cell subsets (memory T cells, T regs), natural killer (NK) cells, with functional markers representing T cell exhaustion (PD-1, LAG-3, TIM-3, PDL-1), activating and inhibitory NK cell receptor (KIR, LIR1, NKG2A, CD57). Relative changes in RNA expressions of target genes related to cancer immunology were evaluated by PCR array.
Results:
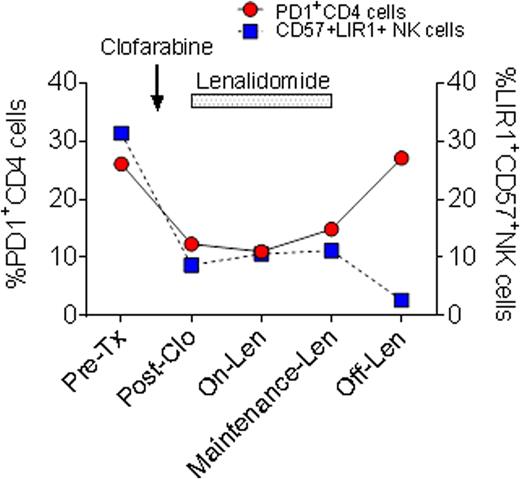
Of four subjects enrolled, two subjects showed responses at 28 days post clofarabine and at 28 days post first cycle of lenalidomide. These two responders tolerated subsequent maintenance lenalidomide for 4-6 months. The other two subjects were non-responders. Three subjects were taken off study due to disease progression on day 70, 162, and 206, and 1 subject for DLT on day 69. The clinical trial is now closed without dose escalation beyond the first cohort for reasons of poor accrual and lack of long term responses. In responders immunophenotype analysis showed a decrease in CD4+ %PD-1 expression in blood or bone marrow (0.87±0.29 fold change) after clofarabine and lenalidomide treatment, rising again after lenalidomide discontinuation in one responder (Figure). In contrast, CD4+ %PD-1 expression was increased in non-responders (1.23±0.19 fold change). In pre-treatment samples terminally differentiated CD57+ NK cell with high LIR1 expression (34.6±23.5%) were increased. In responders, both the levels of CD57 and LIR1 expression fell with reciprocal increase in CD56brightNK cells, suggesting restoration of NK cell repertoires after clofarabine. RNA expression profiles in one responder on maintenance lenalidomide showed significant up-regulation of gene expressions in IL-1A, FASLG, ICAM1, IL-27, WT1 (p<0.01) in comparison to the pre-treatment sample.
Conclusion:
Sequential treatment of low-dose clofarabine and lenalidomide is feasible for elderly patients with high risk MDS and AML. However individuals vary in the lenalidomide dose tolerated. Immune reconstitution profiles supports a potential immune-rebooting effect of clofarabine leading to restoration of CD4 cell and NK cell repertoires with fewer exhaustion or inhibitory markers. Lenalidomide may further promote a favorable anti-leukemic immune milieu. Immune profiling of T and NK cells can help to guide the application of these immune-modifying agents in high risk MDS and AML.
Battiwalla:NIH/NHLBI: Employment.
Author notes
Asterisk with author names denotes non-ASH members.