Abstract
Background
Multiple myeloma (MM) is a cancer of the immune system. Infection is a major cause of morbidity and mortality in MM. Vaccinations (vax) are recommended in MM, but clinical efficacy endpoints have not been demonstrated and surrogate markers of efficacy have limited data. This pilot study evaluated sequential immunologic markers after standard vax for pneumococcal pneumonia (PV) and influenza (FV).
Methods
MM patients and non-MM control (Ctrl) participants signed informed consent to this IRB approved study. The MM patients were not selected for treatment or disease status. Vax was standard PV (PCV13 or PPV23) and/or FV (IIV4) with research laboratory testing at baseline and at 2, 4, 12, 24 weeks after vax. If PV, then PCV13 preferred followed by PPV23 suggested at >= 26 weeks. IgG antibodies (Ab) to influenza and pneumococcal antigens were detected by ELISA. Measurement of Ab to influenza was performed as previously described (Oaks et al. 2013OncoImmunology) and used Flulaval Quadrivalent 2015/2016 form (GlaxoSmithKline) as the coating antigen. Anti-pneumococcal IgG levels were determined with The Binding Site VaccZymeAnti-PCP IgG EIA Kit (#MK012-U) according to the manufacturer's instructions.
Results
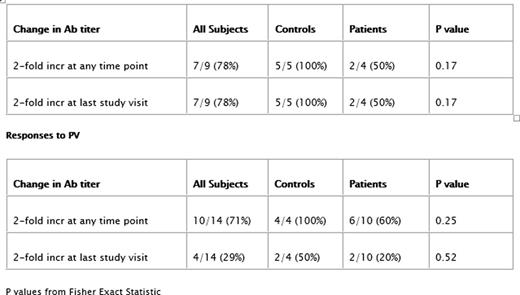
Ten MM (5 M, 5 F) and 9 Ctrl (4 M, 5 F) were enrolled. MM median age was 69.8y (range: 59-78) and Ctrl was 63.5y (range: 56-73). Over the total time course 5/10 MM and 4/9 Ctrl had no missing samples. Vax included: 5 (2 MM, 3 Ctrl) FV only; 10 (6 MM, 4 Ctrl) PV (8 PCV13, 2 PPV23) only; and 4 (2 MM, 2 Ctrl) both FV and PV (3 PCV13, 1 PPV23). Tetanus Ab response was unchanged, which served as a negative control. FV results included 2/4 MM showed at least a doubling of anti-flu titer, although the other 2 showed no measurable response. All 5/5 Ctrl had at least a 2-fold increase in anti-flu titers at some point. PV humoral response varied considerably for both MM and Ctrl. All 4 Ctrl responded with at least a 2-fold increase in Ab titer, only 2 Ctrl had a sustained increase in titer at the closing visit. Six of 10 MM had at least a 2-fold Ab increase at some point during the course of the study. Only 2 MM patients showed a sustained increase of anti-PV Ab. Response rate differences were not statistically significant and there was no relationship between responsiveness to FV or PV and initial serum IgG concentration at entry into the study (data not shown).
Discussion
Study limitations include small sample size, sample dropouts, and heterogeneous population. We are unaware of prior MM vax immunology response time courses. This may be because of the difficulties including PV studies with PCV13->PPV23 recommendations and the potential long follow up. FV response may be more amenable to short term follow up. In 2013 Hahn and colleagues showed proof of principle with "Boost vax improves influenza humoral immune response in MM" (ow.ly/sEBEO), but without a time course or clinical outcomes data. The 2015Branagan et al. (http://ow.ly/W4tLS) pilot study showed a 2 dose FV strategy had lower flu infections compared to historical controls. Karlsson et al. (http://ow.ly/YUumy) showed that Ab response does not correlate with functional assays. We found no relationship with total IgG level. This is consistent with Beers et al. findings that IgG isotypes may be of more importance than the total IgG level. (http://ow.ly/Z5g2A)
Conclusion
We found that FV and PV Ab response was low and not sustained in most MM patients. Infection in MM remains an important concern. Future vax studies should address the following: 1) Homogeneous population - eg therapy trial or autologous stem cell transplant with a standardized re-vax and sequential testing, 2) validate relevant immunologic surrogates - eg Ab response twice baseline, 4) fresh assay vs frozen (batched), 5) correlation of metric with functional outcomes such as hospitalization, cost, and death, 6) and epidemiologic data may complement translational approaches.
Funding
Funding was provided by a Vince Lombardi Cancer Foundation Aurora Cancer Research Award.
Responses to FV
Thompson:Celgene: Membership on an entity's Board of Directors or advisory committees, Other: MDS/AML Registry; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees; AIM Specialty Health: Membership on an entity's Board of Directors or advisory committees; VIA Oncology: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Other: Multiple Myeloma International Registry; Doximity: Equity Ownership, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.