Abstract
Background & Objective
The therapy of multiple myeloma (MM) has improved greatly over the last decade. However, the rise in health care costs has become a big problem. Therefore, it is necessary to consider the cost-effectiveness including Quality of Life (QoL). In this study, we assessed the real-world cost-effectiveness of MM treatment.
Methods & Patients
A total of 209 MM patients who were newly diagnosed and treated only at Japanese Red Cross Medical Center (JRC-MC) from January 2006 to December 2015 were registered for this retrospective research. Those patients who were treated at other institute than JRC-MC were excluded. All of the costs for MM treatment in hematology department were analyzed. The costs were calculated based on the health care costs spent in hematology department. Overall survival was evaluated by the Kaplan-Meier method. All of the costs were calculated in US dollars based on average value from 2006 to 2015 (1USD = 100.294 Japanese yen).
Result
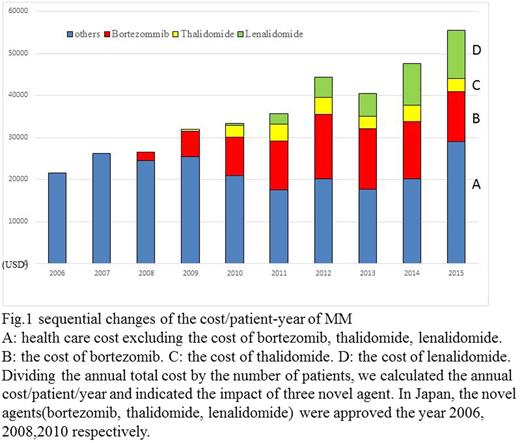
First, the cost paid for treatment of 84 patients who died from symptom onset to death was about 0.14 million USD. We also calculated the total annual health care cost for their patients from 2006 to 2015. Annual cost of all MM patients at 2006 was 0.454 million USD, and the cost at 2015 was 7.505 million USD. The cost/patient-year of 2006, when novel agents were not obtainable was 21,656 USD, and that cost of 2015, when three new agents were available was 55,608 USD. These results showed that the costs of MM treatment became 2.56 times higher in the past 10 years. Individual costs for bortezomib, thalidomide, and lenalidomide is shown in Fig.1. Since 2011 to 2014, the ratio of cost of novel agents exceeded 50%.
Secondly, overall survival was evaluated by statistical analysis. The median survival from diagnosis was 56 (range, 0-117) months 95% CI (45.12-66.88). We divided patients into 5 subgroups, according to the time of diagnosis. (1)2006: 21 patients, (2)2007-2008: 26 patients, (3)2009-2010: 38 patients, (4)2011-2012: 42 patients, (5)2013-2015: 82 patients. In Japan, the novel agents such as bortezomib, thalidomide, lenalidomide were approved the year 2006, 2008, 2010 respectively. Median survival of subgroup (1), (2), and (3) were 56 months 95% CI (42.66-69.33), 55 months 95% CI (19.12-90.87) and 54 months 95% CI (36.42-71.57) respectively. Median survival of subgroup (4) and (5) have not been reached. Then we investigated the treatment costs in these groups. In each subgroup the average annual cost was 332, 518, 507, 588 and 631 USD respectively. Compared to subgroup (1), the cost of other groups were significantly high. These results showed that the impact of three novel agents on medical cost were significant. We also investigated the relationship between health care costs and the length of hospital stay or autologous stem cell transplantation. There were no statistically significant differences among them, however, there was tendency that the length of hospital stay shortened by time; Average length of total hospital stay the subgroup (1), (2), and (3) were 205, 125 and 56 days respectively. Subgroup (4) and (5) showed also shortened length of hospital stays, although observation years subgroup (4) and (5) were short (date was not shown). Among 5 subgroups the cases of received autologous stem cell transplantation were not stastically differences (total 56 patients).
Conclusion
We revealed the real-world costs of MM treatment in a single-institute, by following up individual patients sequentially. Health care costs for treatment of MM increased drastically, by introduction of three novel agents. We also showed that the introduction of three novel agents shorten hospital stay, which leads to improve QoL. Therefore, bortezomib, thalidomide and lenalidomide had the significant impact of QoL. After the further approval of novel agents in Japan, health care costs will be even higher but on the other hand, they might bring positive impacts on OS and QoL. Our study clearly indicated the importance of considering the balance of cost and effectiveness for the MM treatment is important.
Character count: 3477(excluding spaces)
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.