Abstract
Introduction: Identification of patients with high-risk (HR) multiple myeloma (MM) is important to optimise their treatment. In 2015, revised International Staging System (R-ISS) guidelines were published (Palumbo, 2015) where HR cytogenetics (CG) and elevated serum lactase dehydrogenase (LDH) were added to the traditional ISS staging criteria. Thus, R-ISS stage III includes one of the HR CG abnormalities or elevated LDH, ISS stage I patients have no HR CG and normal LDH; the rest of patients belong to R-ISS stage II. There are limited data on the prevalence of R-ISS groups in comparison to the old ISS grouping and impact on clinical outcomes in the real-life setting. The aim of the present analysis was to use structured longitudinal electronic health records (EHR) provided by the Finnish Auria Biobank to compare the prevalence and survival outcome between patients in ISS and R-ISS groups in a real-life patient cohort of 100 patients treated at Turku University Hospital. Auria Biobank covers roughly 15% of the population of Finland and collects samples with the associated data from all diseases treated at Turku University Hospital based on the Finnish Biobank Act.
Methods: Auria Biobank database was analysed retrospectively for all MM-patients diagnosed between 2008-2013 (incident cohort) and whose fluorescence in situ hybridization analysis was performed at the time of diagnosis. Data for age, gender, LDH, creatinine, ISS, R-ISS, CG, time to next treatment and overall survival were collected from Auria Biobank. Classification into ISS groups was done based on data at the time of diagnosis and OS. Classification into R-ISS staging was done according to IMWG (Palumbo, 2015). For HR CG at least one of the following CG abnormalities was required: del(17), t(4:14), or t(14:16). Estimated glomerular filtration rate was calculated by using the CKD-EPI formula. Drug treatments were classified as conventional (e.g. melphalan + prednisolone) or novel (proteasome inhibitors, IMIDs). Descriptive methods and Kaplan-Meier survival analysis were used for comparison of the groups.
Results: The median age of the 100 patients was 64 yrs (range: 37 - 80), and 43% were female. At the time of diagnosis, 17% of patients had high risk CG status, 32% had at least moderate kidney failure (estimated glomerular filtration rate <60ml/min) and 26 % had elevated LDH. 41% patients received autologous stem cell transplant and 64% and 14% were treated with novel and conventional treatments in first line, respectively. 26%, 48% and 26% were classified to ISS stage I, II and III groups respectively, and 21%, 63% and 16% to R-ISS stage I, II and III groups, respectively. Criteria to include patients into R-ISS III were HR CG in 62 %, elevated LDH in 69 % and 31 % fulfilled both criteria. Neither ISS- nor R-ISS staging had any influence on first line treatment decisions between novel and conventional treatments.
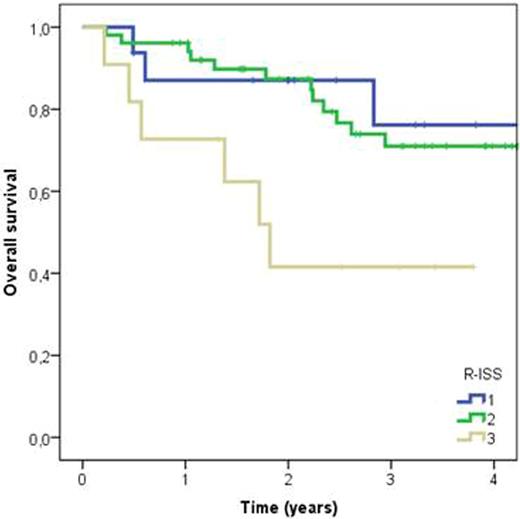
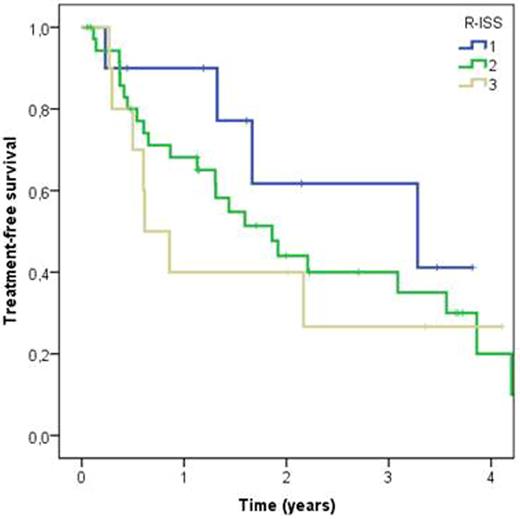
In all patients 2-year OS (from diagnosis) was 82% (median OS not yet reached). The 2-year OS in ISS I, II and III groups was 90%, 88%, and 60% respectively, and in R-ISS I, II and III groups 87%, 85% and 42%, respectively. R-ISS had a statistically significant effect on survival time (log rank P=0.032), with R-ISS III patients having a 3.8-fold risk of death compared to R-ISS I (Fig 1). R-ISS III patients had also shorter (ns) treatment free survival than R-ISS I patients (HR 2.1, log rank P=0.479) (Fig 2). No statistically significant difference was observed between the survival curves stratified by ISS staging groups.
Conclusion: The new R-ISS staging system, using additional information including CG-profile and serum LDH, separated in our real-life setting more profoundly patients with poor prognosis than the old ISS staging. Structured EHRs can successfully be used to derive useful clinical and prognostic data from real-life MM patients.
Kaplan-Meier curves for overall survival in different R-ISS groups. Time is measured from diagnosis to death or end of follow-up. Log rank P=0.032. R-ISS III vs. R-ISS I hazard ratio 3.8.
Kaplan-Meier curves for overall survival in different R-ISS groups. Time is measured from diagnosis to death or end of follow-up. Log rank P=0.032. R-ISS III vs. R-ISS I hazard ratio 3.8.
Kaplan-Meier curves for time to next treatment in different R-ISS groups. Time is measured from beginning of first line treatment to beginning of second line treatment or end of follow-up. Log rank P=0.479. R-ISS III vs. R-ISS I hazard ratio 2.1.
Kaplan-Meier curves for time to next treatment in different R-ISS groups. Time is measured from beginning of first line treatment to beginning of second line treatment or end of follow-up. Log rank P=0.479. R-ISS III vs. R-ISS I hazard ratio 2.1.
Tamminen:Aava Healthcare group: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy; Roche: Employment; Takeda: Employment. Remes:Amgen: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Teva: Membership on an entity's Board of Directors or advisory committees; Janssen-Cilag: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.