Abstract
Background: Histone acetylation plays a key role in regulating gene expression and in control of cellular activities in multiple pathways involved in normal and cancer cell growth.Panobinostat (pano) is a pan histone de-acetylase inhibitor (HDAC-i) approved by the FDA on February 23, 2015 for use withbortezomib (btz) and dexamethasone (dex) for patients with multiple myeloma (MM) who have had at least 2 prior lines of therapy including bothbtz and an immunomodulatory agent (IMiD). The combination ofpano withIMiDs and proteasome inhibitors (PIs) has been found to demonstrate enhanced anti-myeloma activity in clinical trials (Berdeja JG et al, 2015,Haematologica;Mateos M et al, 2010, ASCO Abstract 8030, JCO 28:15s). The goal of this retrospective study is to evaluate the real world experience on efficacy and safety ofpano in combination with a variety of FDA approved agents including a PI, anIMiD or a monoclonal antibody-based regimen in patients with relapsed/refractory MM.
Methods: Between February 23, 2015 and July 1, 2016, 34 consecutive patients with relapsed/refractory MM who were treated with commercialpano were identified from the JohnTheurer Cancer Center. Charts were analyzed for response and safety data. The study was approved by the institutional review board.
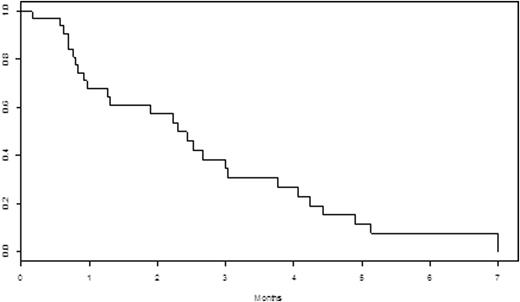
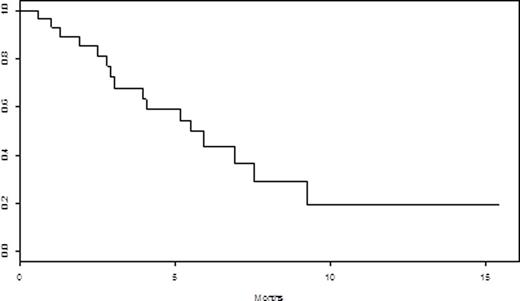
Results: Median age was 63 (range 27-78), with 58% percent men. Thirty-one patients (91.2%) wereDurie-Salmon stage II or III. Ten (30%) had high-risk FISH as defined byt(14;16), t(4;14), del p53, and gain 1q21. Median number of prior lines was 5 (range 2-9). All patients were relapsed/refractory to their last line of therapy, and 18 (53%) werebtz-refractory, 25 (74%) werelenalidomide-refractory, 27 (79%) werepomalidomide-refractory, and 29 (85%) were carfilzomib-refractory. Twenty-five (74%) were refractory to the combination of carfilzomib with anIMiD. Five patients (14.7%) had priordaratumumab, and 4 (12%) had prior HDAC-i therapy. Median number of cycles withpano was 1 (range 1-5). The overall response rate (≥ partial response (PR)) was 23.5% and the clinical benefit rate (≥ minor response (MR)) was 67.6%. The median duration of response (≥ stable disease (SD)) was 3 months. The median progression-free survival (PFS) for all patients was 2.3 months (95% CI: [1.27 - 4.07]). See Figure 1. Median overall survival (OS) from initiation ofpano through 7/27/16 was 5.5 months (95% CI: [3.93, NA]). See Figure 2. Of the 4 patients who were refractory to a prior HDAC-i, 1 achieved PR (4 cycles), 1 achieved MR (5 cycles) and 2 had disease progression. Only 1 patient discontinuedpano due to toxicities. Grade 3 and 4 non-hematologic toxicities were diarrhea (N=1), and hypoxia/respiratory failure (N=1). Grade 3 and 4 hematologic toxicities occurred in 11 (32%) patients, with 5 (15%) anemia, 9 neutropenia (26%), and 8 (24%) thrombocytopenia. Serious adverse events included acute kidney injury, GI bleed, and febrile neutropenia in 3 patients, respectively.
Conclusions: These observations demonstrate that real-world use ofpano outside of the FDA indication in combination with PI andIMiD-based regimens has activity and is well tolerated in heavily pretreated patients with relapsed/refractory MM, even those who have exhausted conventional treatments. Further assessment in a larger prospective study is warranted.
OS of all patients receivingpanobinostat-based regimens from time of initiatingpanobinostat
OS of all patients receivingpanobinostat-based regimens from time of initiatingpanobinostat
Biran:Takeda: Speakers Bureau; Celgene: Speakers Bureau; Novartis: Speakers Bureau; Amgen: Speakers Bureau. Vesole:Janssen: Speakers Bureau; Novartis: Speakers Bureau; Takeda: Speakers Bureau; Celgene: Speakers Bureau; Amgen: Speakers Bureau. Richter:Celgene: Consultancy, Speakers Bureau; Takeda: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Jannsen: Speakers Bureau. Siegel:Celgene: Honoraria, Speakers Bureau; Merck: Honoraria; Takeda: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; BMS: Honoraria, Speakers Bureau; Amgen: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.