Abstract
Background: Autologous stem cell transplantation (ASCT) has been widely used in the treatment of hematological malignancies over the last two decades. Despite its broad use, some characteristics that might influence engraftment have not been exhaustively investigated, particularly graft purity with respect to contamination by platelets (PLTS) and White Blood Cells (WBC). Here we report collection characteristics and engraftment kinetics of a single Center consecutive series of 510ASCTs.
Methods: We retrospectively collected clinical records of patients who underwentleucapheresis procedures (LA; followed or not byASCT) at our Institution over 16 years (2000-2016): 290 patients collected peripheral blood stem cells (PBSC) (80 Multiple Myeloma MM, 133 Non Hodgkin Lymphoma NHL, 22 Hodgkin Lymphoma HL, 32 Acute Myeloid Leukemia AML, 23 other diseases) for a total of 481LAs. Mobilizing regimens are described in Table 1.
We considered the number of harvested CD34+ cells *106/kg the first day of LA. Data on 458 patients (191 MM, 190 NHL, 45 HL, 19 AML and 13 other diseases) for a total of 510 ASCTs were acquired. The impact on engraftment kinetics of conditioning chemotherapies, amount of infused CD34+ cells and WBC/PLTS graft contamination were analyzed. Absolute neutrophil count (ANC) engraftment was defined as the duration of neutropenia (from day 0 to the first of 3 consecutive days of ANC>500/ul post ASCT).
Results: Regarding CD34+ cell collection, no impact of mobilizing regimens and WBC count during LA was observed. On the other hand, we observed a difference in the number of total CD34+ cells collected among different diagnoses: the median overall collection was 7.2 (0.65-64.06)*106/kg CD34+ cells for NHL patients, 5.66 (0.71-23.31)*106/kg for MM patients, 6.15 (0.51-23.24) *106/kg for HL patients and 3.56 (0.64-20.3)*106/kg for AML patients) (p = 0.001). Considering CD34+ cells/kg harvested on the first day of LA, 59.2% of NHL and HL, 57.5% of MM patients and 34% of AML patients harvested ³ 5*106/Kg CD34+ cells. Of note, among AML patients, 40.6% collected less than 2.5*106/kg. The differences were statistically significant (p = 0.003) (Tab. 2). Moreover, an inverse correlation between collected CD34+ cells and age was shown (p = 0.001) (Fig.1).
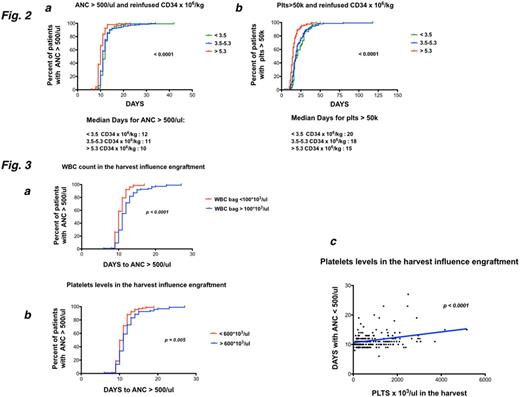
ANC recovery after ASCTwas not influenced by conditioning regimen whereas diagnosis impacted on the duration of neutropenia (AML patients displayed a longer aplasia, p < 0.01). We observed that the median days with ANC<500/ul were 10, 11 and 12 in patients who received >5.3*10^6/kg, 3.5-5.3*10^6/kg and <3.5*10^6/kg CD34+ cells, respectively (p <0.0001) (Fig 2a). Furthermore, the same finding was observed considering the duration of thrombocytopenia (median number of days with PLTS <50000/ul: 15, 18 and 20 in patients who received >5.3*10^6/kg, 3.5-5.3*10^6/kg and <3.5*10^6 CD34+ cells, p<0.0001) (Fig.2b).
Looking at the apheresis product, we analyzed the impact of harvest contaminating WBC and PLTSon engraftment kinetics. Notably, when the ASCTcollection contained >100*103/ul WBC, ANC engraftment (days with ANC < 500/ul) lasted longer (median days 11) compared to patients who received a graft with lower WBC count (p < 0.0001) (Fig. 3a). A faster ANC engraftment was also observed in patients receiving harvests with PLTS levels >600*103/ul compared to those who infused a collection bag with PLTS <600*103/ul (p = 0.005) (Fig.3b,c).
Conclusions: Herein, we confirmed that the disease and the amount of infusedCD34+ cells significantly influence time of ANC andPLTS engraftment; furthermore, we observed for the first time that quality and purity of the graft have a substantial impact on engraftment kinetics.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.