Abstract
INTRODUCTION: Despite advancements in induction and maintenance therapies leading to improved response rates and overall survival (OS), virtually all patients with multiple myeloma (MM) eventually relapse. Improvement in outcomes in relapsed and/or refractory multiple myeloma (RRMM) remains an area of unmet need, yet there is a shortage of data describing typical treatment patterns in these patients in real-world practice settings. Such data may help inform future health technology assessments and other regulatory evaluations of current and new therapies in RRMM. To help address this information gap, we analyzed retrospective data from a cohort of RRMM patients in the United Kingdom (UK).
METHODS: A retrospective medical record review was conducted in a cohort (n = 216) of patients with RRMM in the UK. Patients were selected (based on randomly generated first letter of last name) from the caseloads of 41 hematology/oncology providers across the UK. Specific inclusion criteria were: ≥18 years of age at initial MM diagnosis; first determined to have RRMM between January 1, 2009 and December 31, 2011, where RRMM was defined by (1) receipt of a first-line (induction) treatment regimen of chemotherapy with or without stem cell transplant (SCT) and with or without post-induction/SCT maintenance therapy and (2) disease progression while on or at any time after completion of first-line therapy. Patients were retrospectively assessed on second- and third-line treatment regimens received, treatment duration, and reasons for treatment discontinuation from date of first relapse or progression (study index date). All analyses were descriptive and exploratory in nature.
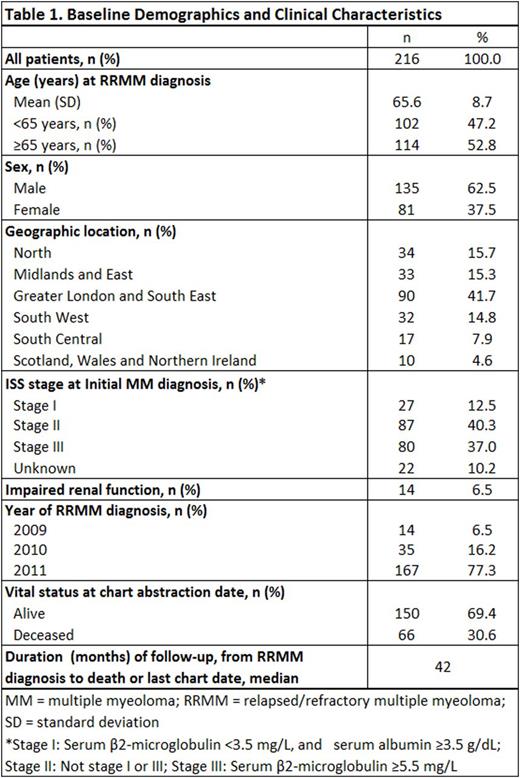
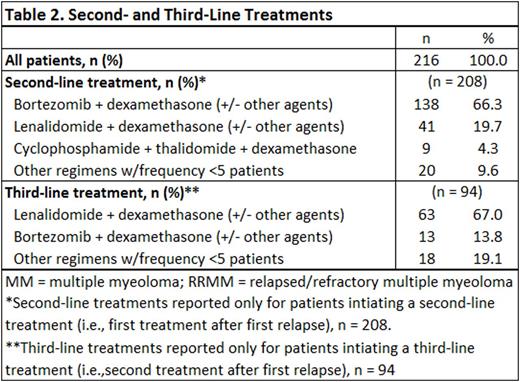
RESULTS: Demographic and clinical characteristics of the study sample are presented in Table 1. Mean (SD) age at study index (first relapse, or RRMM diagnosis) was 65.6 (8.7) years, with approximately half of patients (53%) having advanced age (≥65 years). The study sample was 62.5% male and more than two-thirds of patients (69%) were still alive at the time of the medical record review. Among the 216 patients studied, 208 (97%) received ≥1 additional line of systemic chemotherapy after first relapse (Table 2); 94 patients (43%) received ≥2 additional lines of therapy (i.e., at least second- and third-line therapy) during the observation period. The most common second-line regimen was bortezomib + dexamethasone with or without other agents (66% of second-line initiators), followed by lenalidomide + dexamethasone with or without other agents (20%). Median duration of second-line treatment was 6 cycles over a median of 5.4 months. Among the 98% of patients who discontinued second-line treatment, a majority (62%) stopped therapy due to reaching complete response with no additional benefit expected; 33 patients (16%) discontinued second-line treatment due to disease progression and 8% discontinued due to toxicities. Lenalidomide + dexamethasone with or without other agents was the predominant third-line regimen (67% of third-line initiators); bortezomib + dexamethasone with or without other agents was the next most common third-line regimen (14%). Among the 94 patients receiving third-line treatment, median duration was 6 cycles over a median of 5.7 months. The leading reason for third-line discontinuation was disease progression (48%); 30% of patients discontinued because they reached complete response with no anticipated additional benefit, and 20% discontinued because of loss or lack of response to therapy.
CONCLUSIONS: In the RRMM cohort reviewed here, bortezomib-containing regimens were the predominant second-line therapy and lenalidomide + dexamethasone was the most common third-line regimen. The most common reasons for discontinuation of RRMM treatments were disease progression and physician-judged achievement of complete response with no additional benefit expected. While the most common reason for therapy discontinuation in second-line treatment was reaching complete response with no additional benefit expected, in third-line therapy it was disease progression. With growing evidence in the RRMM literature that treatment to progression may be superior to a fixed duration of therapy, as well as evidence of premature discontinuation being associated with inferior outcomes, the relatively short second-line duration reported here (<6 months) further highlights a potential unmet need in this disease area.
Lin:Takeda: Employment. Davis:Takeda: Research Funding. Kaye:Takeda: Research Funding. Luptakova:Takeda Oncology: Employment. Nagar:Takeda: Research Funding. Seal:Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.