Abstract
Background
The dose of many anti-cancer medications is calculated based on patient weight or body surface area (BSA). This patient-specific method means that there is almost always leftover drug in the vial which is then discarded. This has been identified as a significant source of wastage and a contributor to the increasing cost of cancer treatment. Bortezomib is a first in class proteasome inhibitor approved for the treatment of multiple myeloma (MM) both at front line and relapse.
The starting dose for myeloma is 1.3mg/m2 and the only available preparation in the UK is a vial containing 3.5mg of bortezomib. The list price is GBP 762 ($1,006) per 3.5mg vial. The physical and chemical stability of the reconstituted drug has been demonstrated for 21 days in the original glass vial and in a syringe thereby allowing the preparation of doses in advance of a patient attending for treatment. We carried out a single centre retrospective analysis of the use of bortezomib in patients with MM, with vial sharing to minimise wastage, with focus on practicality and cost saving.
Methods
Between 27/04/2015 and 15/05/2016 we prepared all scheduled doses of bortezomib in one of two batches each week (changed to a single weekly batch from 11/10/2015) thereby enabling us to share vial contents between patients and minimise drug wastage.
Planned bortezomib doses were identified from the hospital electronic prescribing system and ordered from the pharmacy on the Friday of the week prior to treatment.
Dispensing occurred under aseptic conditions in the pharmacy sterile production unit using worksheets generated directly from the prescribing system. All doses were prepared for administration by subcutaneous bolus injection as a 2.5mg/ml dilution in sodium chloride 0.9%.
The average cost per bortezomib dose was retrospectively calculated by dividing the total acquisition cost of the vials used by the number of doses administered and this was then compared with the cost of using one vial per dose. The cost of wasted doses (those prepared in a batch but not subsequently administered) and doses prepared individually (separately, in addition to the batch) were included to provide a real world assessment of the impact on cost.
Results
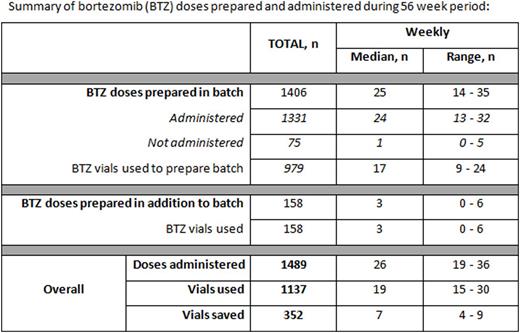
During the 56 week audit period 1489 bortezomib doses were administered to 120 patients, median 26 doses per week (range 19-36), of these, 1331 (89% of total administered) were prepared in one of the vial-sharing batches. The mean actual prescribed dose of bortezomib was 2.36mg (range 1.4-3.0mg).
The total number of doses prepared in the batch but not subsequently administered was 75 (5.3%), median 1 per week (range 0-5).
Of note, unused doses did not always contribute to drug wastage if an additional vial had not been necessary to accommodate this dose in the batch (27 out of 75 doses).
The reasons for unused doses were: individual dose delayed (due to toxicity (n =27), social reason/patient request (n=13), or other reasons (n=7)), bortezomib treatment stopped (due to disease progression (n=12) or toxicity (n=8)) or dose ordered in error for a patient known to have stopped treatment or been delayed (n=8).
The total number of doses prepared in addition to the batch was 158, median 3 per week (range 0-6). These doses were for patients who had not been included in the batch due to them not being prescribed in advance (n = 99) or who were missed during the ordering process (n= 59).
A total of 1137 vials of bortezomib were used to dispense the doses, of which 979 were used for batched preparation (see table for summary). The median total number of bortezomib vials used each week was 19 (range 15-30) and number of vials saved each week was 7 (range 4-9).
Batch preparation and vial-sharing reduced the total cost of bortezomib vials needed from GBP 1,134,618 ($1,497,696) to GBP 866,394 ($1,143,640) representing an overall saving of GBP 268,224 ($354,056). The effective cost of a single bortezomib dose was reduced by 23.6% from GBP 762 ($1,006: the cost of using one vial per dose) to GBP 582 ($768).
Batch preparation also reduced dispensing time in the pharmacy and patient waiting times on the day unit as doses were prepared in advance of the day of treatment.
Conclusions
Sharing the contents of bortezomib vials between patients is logistically feasible and improved patient experience by reducing waiting times on treatment days. Drug costs were reduced by 23.6% resulting in significant savings. This approach should be explored for other suitable drugs.
Cheesman:Janssen: Consultancy. Yong:Autolus Ltd: Equity Ownership, Patents & Royalties: APRIL based chimeric antigen receptor; Janssen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.