Abstract
Introduction
Hepatic veno-occlusive disease, also called sinusoidal obstruction syndrome (VOD/SOS), is a potentially life-threatening complication of conditioning for hematopoietic stem cell transplant (HSCT), and its severity can be variable in its course over time. VOD/SOS with multi-organ dysfunction (MOD)/multi-organ failure (MOF) may be associated with >80% mortality. In the European Union, defibrotide is approved for the treatment of severe hepatic VOD/SOS post-HSCT. Defibrotide recently was approved for treating hepatic VOD/SOS with renal or pulmonary dysfunction post-HSCT in the United States (US). In the US, defibrotide had been available through an expanded-access program to generate the largest experience of its kind (n >750). The optimal time to initiate VOD/SOS treatment with defibrotide is an area of active investigation, with previous studies suggesting that earlier intervention is associated with better outcome.
Methods
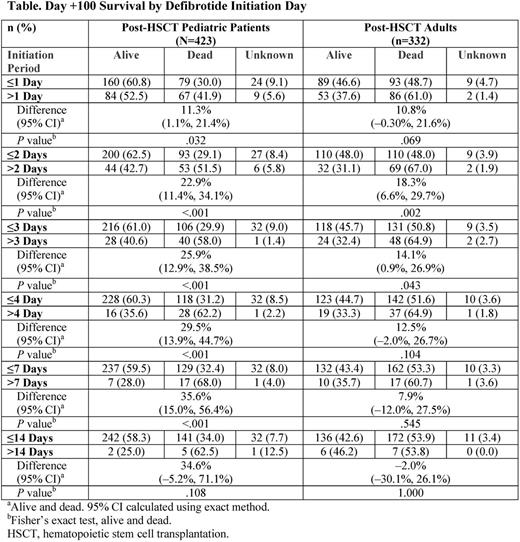
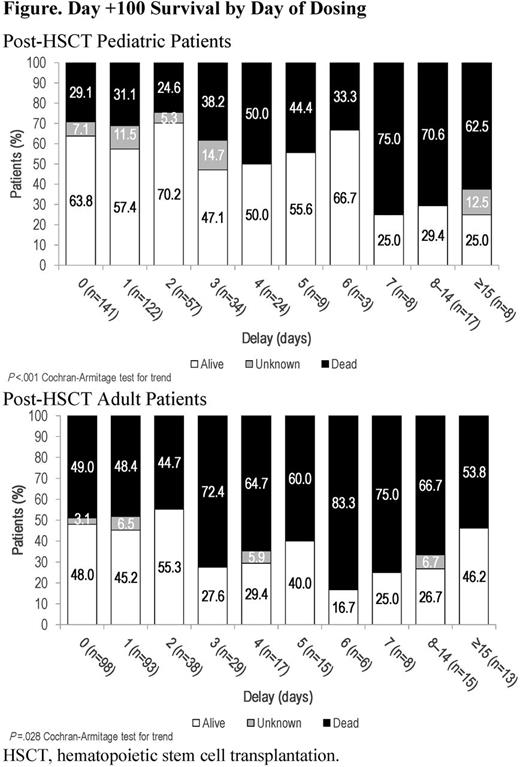
Patients with VOD/SOS (by Baltimore or modified Seattle criteria, or biopsy), with or without MOD/MOF (renal and/or pulmonary dysfunction) were eligible for the expanded-access program. Defibrotide 25 mg/kg/day was given in 4 divided doses for a recommended ≥21 days. Here, Day +100 survival post-HSCT in pediatric (age ≤16 years) and adult patients were explored post hoc based on time from VOD/SOS diagnosis to initiation of defibrotide. There were 2 analyses: (1) survival rate analyzed by treatment initiation day for all post-HSCT patients in each age group before or after days 1, 2, 3, 4, 7, and 14, using Fisher's exact test; (2) survival rate by age group for only those patients with treatment initiated on a particular day (0, 1, 2, 3, 4, 5, 6, 7, 8-14, and ≥15), with a Cochran-Armitage test for trend across days.
Results
Treatment initiation data were available for 755 post-HSCT patients enrolled through April 18, 2015, including 423 pediatric patients (232 with MOD) and 332 adults (194 with MOD). In the pediatric and adult groups, defibrotide was started on the day of diagnosis in 33% of pediatric and 30% of adult patients respectively, and 94% and 92%, received defibrotide within 7 days post-diagnosis.
In the analysis of treatment initiation before or after days 1, 2, 3, 4, 7, and 14 across each age group, earlier initiation of defibrotide was associated with higher survival rates for all cut-points except Day 14, with only 2% of pediatric and 4% of adult patients beginning treatment post-day 14 (Table). In the analysis of the relationship between Day +100 survival and treatment initiation day, there was a statistically significant trend over time for higher Day +100 survival with earlier initiation both in pediatric patients (P<.001) and adults (P=.028; Figure). In pediatric and adult patients with MOD/MOF, survival was lower than in the overall cohorts, but patterns of better outcomes with earlier treatment were generally similar to those in the overall VOD/SOS age groups.
Conclusions
In this post-hoc analysis, decreased Day +100 survival in pediatric and adult groups was associated with longer treatment delays post-VOD/SOS diagnosis, confirmed by Cochran-Armitage testing. These results suggest that irrespective of age, early defibrotide initiation post-VOD/SOS diagnosis may improve Day +100 survival outcomes, although no specific day post-diagnosis provides a clinically meaningful cutoff for better outcome, suggesting that later intervention still retains value if therapy is not initiated sooner.
Support: Jazz Pharmaceuticals.
Grupp:Jazz Pharmaceuticals: Consultancy; Pfizer: Consultancy; Novartis: Consultancy, Research Funding. Kernan:The National Cancer Institute of the National Institutes of Health: Research Funding; Gentium: Research Funding. Antin:Gentium SpA/Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Liang:Jazz Pharmaceuticals, Inc.: Employment, Other: stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals plc. Hume:Jazz Pharmaceuticals, Inc.: Employment, Other: stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals plc. Tappe:Jazz Pharmaceuticals, Inc.: Employment, Other: stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals plc. Richardson:Jazz Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.