Abstract
Background:Little is known about the frequency of cognitive impairment in older patients with blood cancers, nor how such impairment may contribute to their overall frailty. Understanding the prevalence of impairment is specifically important for this population, because intact cognition is needed to make complex medical decisions, including whether to pursue intensive treatments versus supportive care. We report data from the Older Adult Hematologic Malignancy (OHM) clinic at the Dana-Farber Cancer Institute, which routinely screens patients 75 or older for frailty using tools that incorporate subjective and objective measures of cognition.
Methods: Since February of 2015,all patients aged 75 and older who present for initial consultation for hematologic malignancy have been approached for evaluation by a trained clinic assistant. In a 15-minute interview, the assistant uses two parallel methods of screening to characterize the patient as frail, pre-frail or robust. The first employs a "cumulative deficit" approach (Rockwood, 2007) including a 26-item questionnaire adapted from theYale Precipitating Events Project (Searle, 2008), a grip strength test (Gill, 2006), gait speed test (Studenski, 2011), delayed recall section of the Montreal Cognitive Assessment (MOCA; Nasreddine, 2005) and the Clock In Box test, a cognitive impairment screening tool (CIB; Chester, 2011). The second uses a "phenotypic" approach (Fried, 2001), which gives equal weight to the gait speed and grip strength tests, plus three questions about weight loss, energy expenditure and self-reported exhaustion. We also assess two subjective measures of cognition: (1) whether the patient knows why s/he is presenting to the hospital and (2) whether s/he needs help filling out the questionnaire.
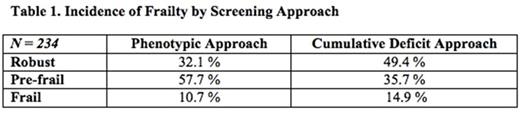
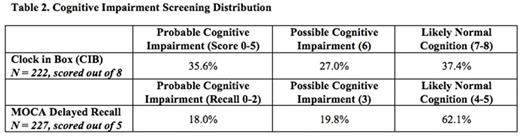
Results: As of July 1, 2016, 235 of 275 patients approached (85%) agreed to be assessed. Median age was 78 years and 37% were female; 32% were seen in the leukemia clinic, 37% in the lymphoma clinic and 31% in the myeloma clinic. More patients were determined to be definitively frail or robust by the cumulative deficit approach compared with the phenotypic approach, which categorized the majority of patients as pre-frail (Table 1). With respect to cognition, 35.4% of the cohort was found to have probable impairment and 27.3% possible impairment by CIB (Table 2). Fewer patients were found to have probable impairment (18.0%) and possible impairment (19.8%) by MOCA delayed recall. Overall, 18.5% of robust patients had possible or probable cognitive impairment by CIB, and 9.5% by MOCA delayed recall. Nearly 7% of the patients could not explain why they were presenting to the hospital; all of these patients also had probable cognitive impairment and were pre-frail or frail. Moreover, 17% of the total cohort needed assistance completing the questionnaire; half of these had probable cognitive impairment, 43% had possible impairment, and most (92.5%) were pre-frail or frail. The odds of being pre-frail or frail (phenotypic) if cognitively impaired per CIB was 1.89 (95% CI 1.073.34; p=0.03). The odds of being pre-frail or frail (phenotypic) if cognitively impaired per MOCA delayed recall was 1.86 (95% CI 1.02-3.40; p=0.04).
Conclusions: Cognitive impairment was prevalent in this cohort of older patients with blood cancers, with between one- to two-thirds of patients testing as probably or possibly impaired using standard measures.A portion of patients who were robust per the brief phenotypic frailty screen were also cognitively impaired, which suggests routinely performing comprehensive frailty screening (cumulative deficit) or adding a cognitive measure such as the CIB. Compared to the phenotypic approach, the cumulative deficit approach, which incorporates specific measurement of cognitive impairment, seemed to result in further discriminaton between patients who were frail versus robust. The CIB test detected more cognitive impairment than the MOCA delayed recall, likely because it assesses both executive function and working memory, whereas the MOCA delayed recall only tests the latter. Taken together, our data suggest that cognitive impairment likely contributes significantly to overall frailty for older patients with blood cancers, and argue for routine testing for such impairment in this patient population.
Stone:Amgen: Consultancy; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jansen: Consultancy; Seattle Genetics: Consultancy; Celator: Consultancy; Agios: Consultancy; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy; Novartis: Consultancy; Pfizer: Consultancy; ONO: Consultancy; Juno Therapeutics: Consultancy; Merck: Consultancy; Roche: Consultancy; Sunesis Pharmaceuticals: Consultancy; Xenetic Biosciences: Consultancy. Soiffer:Kiadis: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Juno: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.