Abstract
[Background] The frequent loss of heterozygosity of the HLA haplotype in the short arm of chromosome 6 (6pLOH) in leukocytes is thought to offer compelling evidence of cytotoxic T cell (CTL) involvement in the development of acquired aplastic anemia (AA) because it represents the escape of hematopoietic stem/progenitor cells (HSPCs) with 6pLOH from the attack of CTLs that are specific to autoantigens presented by the lacked HLA class I allele. Although our previous study suggested that HLA-B*40:02 is the major allele involved in this phenomenon, the exact role of B*40:02 remained unclear because 6pLOH involving this allele is always associated with a lack of HLA-A and C alleles in the haplotype, and the presence of B*40:02-missing leukocytes were unable to be shown due to the lack of monoclonal antibodies (mAbs) specific to B61, the HLA-B antigen that corresponds to B*40:02. We recently succeeded in generating a mAb specific for HLA-B61 that enabled us to explore the role of B*40:02 in the development of AA.
[Methods] Using the new mAb, we examined peripheral blood samples of 28 AA (12 with 6pLOH and 16 without 6pLOH) patients carrying this allele for the presence of B61(-) leukocytes using flow cytometry. HLA genes were enriched by sequence capture, a hybridization-based gene enrichment method, from genomic DNA of sorted B61(-) granulocytes, and were subjected to deep sequencing using an NGS (MiSeq). B61(+) granulocytes or T cells were used as controls. Potential mutations responsible for the B61-missing were identified when 10 or more variant reads were found only in B61(-) granulocytes. Thereafter, HLA-B alleles carrying those mutations were determined taking advantage of the nearest allele-specific SNPs.
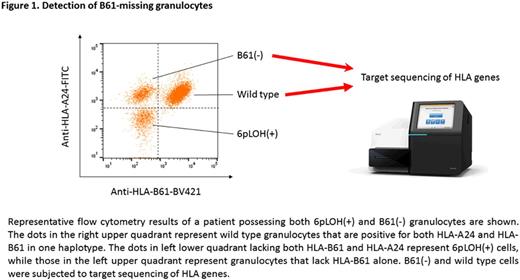
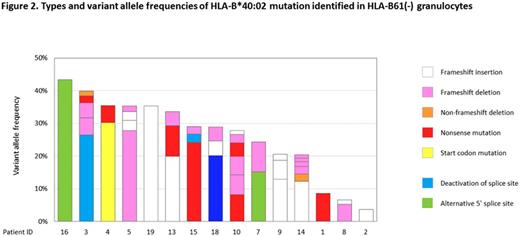
[Results] Among the 12 6pLOH(+) patients, 10 (83%) possessed 0.5%-60% B61-missing granulocytes that were not lacking HLA-A, in addition to 12% to 99% 6pLOH(+) granulocytes that lacked both B61 and an HLA-A allele on the same haplotype (Figure 1). B61(-) granulocytes that accounted for 0.5%-99% of the total granulocytes were detected in 9 (56%) of the 16 6pLOH(-) patients. The prevalence of missing B61 in the 28 AA patients was 21/28 (75%), much more frequent than those of the 3 other major alleles (A*02:01, 32%; A*02:06, 30%; A*24:02, 6%). B61(-) granulocytes were available for mutation analyses of HLA-B alleles in 15 of the 19 patients who possessed B61(-) granulocytes. The mean coverage of HLA-B gene was 426x. In total, 43 somatic mutations of HLA-B were identified in B61(-) granulocytes, all of which were present in B*40:02 but not in any of the other HLA-B alleles. Median variant allele frequency was 4.8% (range, 1.0% - 43%) and the number of mutations in each patient was 1 to 6 (Figure 2). Thirty-nine mutations were exonic while 4 were intronic. Exonic mutations included frameshift insertions (n=12), frameshift deletions (n=16), non-frameshift deletions (n=2), nonsense mutations (n=7), a missense mutation (n=1) and a start codon mutation (n=1). All four intronic mutations were considered to be a splice site mutation; two mutations deactivated 5' and 3' splice sites, whereas the other two were single base substitutions within intron 3, making alternative 5' splicing site with strong consensus sequence: GGC [A>G] TGAGT and TTC [C>G] TGAGT. Surprisingly, missense mutations in the alpha-2 and alpha-3 chain-coding region of HLA-B*40:02 were detected exclusively in the B61(+) granulocytes of two patients possessing B61(-) granulocytes, suggesting the inability of the mutant HSPCs to interact with CTLs. Variant allele frequencies of the two missense mutation were 40% and 45%, respectively. As a result of the mutation, virtually all granulocytes of the two patients were affected by B*40:02 mutations that allowed the HSPCs to escape the T cell attack.
[Conclusions] The markedly high prevalence of leukocytes lacking HLA-B*40:02 as a result of either or both 6pLOH or structural gene mutations clearly indicates that antigen presentation by HSPCs to CTLs via the HLA-B allele plays a critical role in the pathogenesis of AA.
Takamatsu:Celgene: Honoraria; Janssen Pharmaceuticals: Honoraria. Nakao:Alexion Pharmaceuticals: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.