Abstract
PIM kinases, a family of serine/threonine kinases, play an important role in regulating cell survival, cell proliferation, transcription activation and protein translation. Of the three isoforms, PIM-1, PIM-2 and PIM-3, some of them are overly expressed in several hematological malignancies as well as solid tumors. Thus, these kinases have been targeted clinically in several cancer studies. However, the roles of PIM kinases in T cells from previous studies are inconclusive and the functions of each isoform in these cells are yet to be investigated.
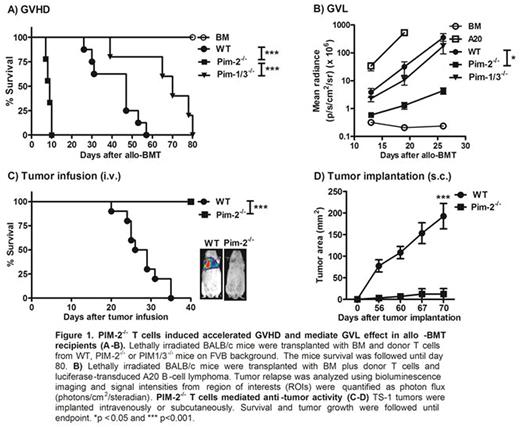
In our study, we focused on the role of PIM-2 kinase in T cell responses to allo-antigen and tumor using pre-clinical models. Using allogeneic bone marrow transplantation (allo-BMT) models, we transferred allogeneic T cells isolated from mice deficient for Pim-2 (Pim-2-/-, FVB background) in comparison to WT and PIM1/3double deficient mice (PIM1/3-/-) into lethally irradiated Balb/C recipients. Our data revealed that upon allogeneic stimulation, PIM-2-/- T cells induced accelerated graft-versus-host-disease (GVHD) compared to WT or PIM1/3-/- T cells (Fig.1A). In addition, PIM-2-/- donor T cells were highly activated with significantly increased proliferation and IFN-γ production in recipient spleen and GVHD target organs. Donor PIM-2-/- T cells also expressed high levels of chemokine receptor, CXCR3 and a4b7 integrin (gut homing receptor) that correlated with increased capacity of donor T-cell migration to GVHD target organs such as liver and gut. In graft-versus-leukemia (GVL) study, PIM-2-/- donor T cells had roughly 4-fold higher ability to mediate GVL effect to WT and PIM1/3-/- T cells (Fig.1B). Negative regulation of T-cell responses to allo-antigens by PIM-2 was independently verified by restoring PIM-2 expression on PIM-2-/- T cells or inhibiting PIM kinases in PIM1/3-/- T cells. Altogether, the data from allo-BMT studies suggested that PIM-2 isoform functions as a negative regulator in T cell allo-response in contrast to PIM-1 and PIM-3 kinases.
We then focused on evaluating T-cell immunity against syngeneic tumor. By administering tumor (TS-1 breast tumor in FVB background) into WT and PIM-2-/- mice either subcutaneously or intravenously, we observed that PIM-2-deficient mice efficiently control tumor growth in sharp contrast to WT mice (Fig. 1C). Mechanistic study revealed that tumor rejection in PIM-2-/- recipient relies on CD8+ tumor infiltrating lymphocytes (TILs) with a significant up-regulation of IFN-g, TNF-a and Fas-L expression. Lower infiltration of regulatory T cells and down regulation of PD-1 expression on T cells recovered from tumor site were also observed in PIM-2 deficient mice. Moreover, the tumor growth rate in PIM-2-/- mice after T-cell depletion with antibody was similar to that of WT mice. To further exclude the effect of other immune cells involved in tumor regression, we adoptively transferred WT and PIM-2-/- CD8+ T cells into WT mice with pre-established TS-1 tumor. CD8+ T cells deficient for PIM-2 was more efficient in controlling of tumor growth than WT or PIM1/3-/- CD8+ T cells. These data indicate that inhibition of PIM-2 in T cells display superior anti-tumor activity. In conclusion, PIM-2 kinase possesses a distinct role in anti-tumor immunity compared to the other two isoforms and it represents a potential target to enhance anti-tumor activity of adoptive T-cell immunotherapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.