Abstract
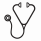
Background: Low-molecular weight heparins (LMWH) are not traditionally used to treat venous thromboembolism (VTE) among dialysis patients because their renal clearance may lead to less predictability in the degree of anticoagulation for a given dose. We determined the risk of major bleeding with LMWH compared with vitamin K antagonist (VKA) use in dialysis patients diagnosed with VTE in a real world setting.
Methods: Using the linked administrative healthcare databases of the province of Quebec, Canada, we identified all patients with incident VTE between 2000 and 2009 and pre-existing dialysis status (hemodialysis or peritoneal dialysis). We included patients dispensed VKA or LMWH for the first time within 30 days of their VTE. Monotherapy was successive dispensation of LMWH in the absence of VKA, or vice versa. Combination therapy was LMWH and VKA on the same day. For each patient, we determined average daily dose and duration of continuous use. Patients were followed from VKA or LMWH initiation until a switch or discontinuation of anticoagulation, or major bleeding (emergency room visit or hospital admission for gastrointestinal bleeding or bleeding into a critical organ). Using a Cox proportional hazards model, we determined the incidence of major bleeding associated with LMWH monotherapy, compared with VKA monotherapy. The risk estimate was adjusted for deciles of a propensity score for LMWH use, which included comorbidities-, dialysis-, and VTE-related covariates, as well as calendar year.
Results: In all, 647 dialysis patients with VTE were identified: 467 started VKA, 82 started LMWH, and 96 started both. Initiators of LMWH were 35 dalteparin, 26 tinzaparin, 19 enoxaparin, and 2 nadroparin. Median (interquartile range, IQR) daily doses were 12,500 (7,500-17,570) IU dalteparin, 16,080 (13,540-20,000) IU tinzaparin, 100 (70-120) mg enoxaparin, and 15,910 (15,200-16,625) IU nadroparin. Median (IQR) duration of LMWH monotherapy was 37 (22-87) days, and 132 (65-235) for VKA monotherapy. More than 90% of LMWH monotherapy was from 2004 and onwards, and 80% of LMWH users had cancer (Table). There were 22 major bleeding events (86% gastrointestinal), 20 in VKA and 2 in LMWH users. No fatal bleeding occurred. Compared with VKA monotherapy, LMWH monotherapy was not associated with major bleeding (adjusted HR, 1.21; 95% CI: 0.20-7.37).
Conclusions: To our knowledge, this is the first population-based cohort reporting LMWH use in dialysis patients with VTE. We observed LMWH monotherapy mostly in cancer patients and from 2004 and onwards. This may reflect the evidence supporting improved effectiveness of LMWH compared with VKA for cancer-associated VTE. Further, daily doses of LMWH were generally halved. Overall, LMWH alone in dialysis patients was not associated with an increased major bleeding risk. If replicated, these findings indicate LMWH use is feasible in this clinical setting.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract