Abstract

The red blood cell (RBC) storage lesion reflects cumulative damage to stored erythrocytes. Randomized clinical trials have yet to identify clinical consequences associated with prolonged RBC storage, however the majority of these trials have not incorporated dose-response analyses. In one of the subgroup analyses undertaken in the ABLE trial, a potential volume-dependent effect associated with storage was identified1.
In this secondary analysis of the ABLE study, we conducted detailed analyses aiming to better characterize whether the number of RBC units administered to patients receiving either fresh or standard-issue blood is associated with mortality in critically-ill adults.
We undertook a secondary evaluation of the 2510 patients enrolled in the ABLE trial. This randomized, controlled, clinical trial compared mortality rates and several secondary outcomes in mechanically-ventilated adults, who received either "fresh" RBC units (stored for ≤7 days) or "standard-issue" RBC units (first-in, first-out). Our secondary analysis consisted of comparing short- and long-term hazards of death between fresh and standard arms within pre-specified subgroups of patients that received ≤3 RBC units and those that received >3 units. Additional analyses using subgroups defined by 2-unit, and 1-unit increments were performed. Cox proportional hazards regression was used to model the hazard of death at the specified endpoints, adjusting for possible confounders. We examined interactions between randomization to fresh RBCs and number of RBC units transfused, as a means of establishing a dose-dependent relationship. We also undertook adjusted and unadjusted survival analyses between treatment arms within subgroups.
In the subgroup that received ≤3 RBCs, there were 754 patients in the fresh arm and 789 in the standard arm; among patients transfused >3 RBCs, there were 457 in the fresh arm and 430 in the standard arm. When stratified by 2 unit increments, in the fresh and standard arms, respectively, there were 601 and 632 patients given ≤2 units, 279 and 272 given 3-4 units, 108 and 107 given 5-6 units, and 223 and 208 given ≥7 units.
Baseline characteristics were comparable between treatment arms within major subgroups, aside from a slightly higher proportion of fresh RBC patients admitted due to trauma in the most transfused subgroups (>3 units: 11.4% of standard, 16.4% of fresh; ≥7 units: 11.5% of standard, 19.3% of fresh) and fewer patients admitted with medical causes in the same subgroups (>3 units: 77.7% of standard, 69.6% of fresh; ≥7 units: 77.9% of standard, 67.7% of fresh).
There was significant interaction between fresh RBC unit transfusion and the volume of transfused RBC units on long-term (90 and 180 days) but not short-term (28-day, in-ICU, in-hospital) hazards of death for all subgroup analyses. This observation suggests that the effect of RBC storage differs with different transfusion volumes.
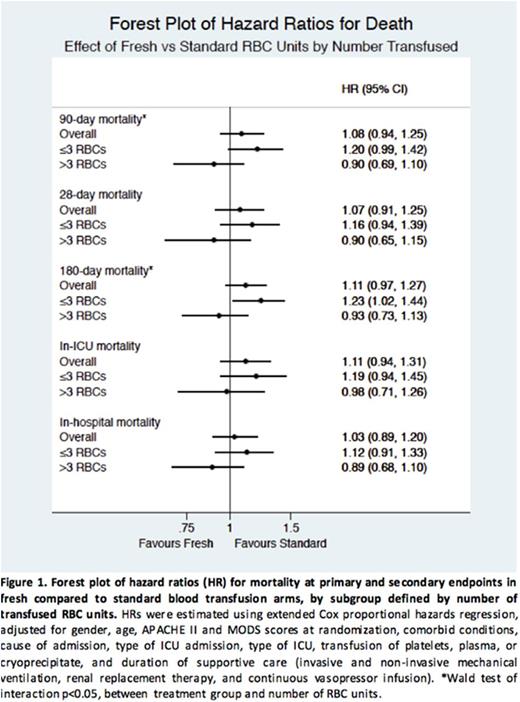
In subgroups of ≤3 and >3 units, a trend was observed of increased hazard of death with fresh RBCs in the less-transfused subgroup, and decreased hazard of death with fresh RBCs in the more-transfused subgroup at all endpoints (Figure 1). Similar trends were observed with smaller RBC unit increments. At all endpoints, the hazard of death was higher with fresh RBCs in patients given 3-4 units and, after a threshold of 5 units, the trend reversed: patients given ≥7 fresh units had a lower hazard (Figure 2).
Overall survival was not significantly different between treatment arms in any of the subgroups.
This secondary analysis suggests a volume-dependent, threshold effect of prolonged red cell storage. Fresh RBCs were associated with a trend of decreased hazard of mortality when more than 5 units were transfused. The findings support current transfusion practice for patients requiring transfusion of <5 RBC units. Further study on the effect of RBC unit storage age should be focused on patients anticipated to require more than 5 RBC transfusions.
The ABLE trial was funded by the Canadian Institutes of Health (grant #177453) and the Établissement français du sang. Dr. Mack is supported by a Fonds de recherche du Québec - Santé 'Resident Physician Health Research Career Training Program' award.
1. Lacroix J, Hébert PC, Fergusson DA, Tinmouth A, Cook DJ, Marshall JC, Clayton L, McIntyre L, Callum J, Turgeon AF, et al. Age of Transfused Blood in Critically Ill Adults. New England Journal of Medicine. 2015;372(15):1410-1418.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract