Abstract
Multiple causes (pseudothrombocytopenia, hemodilution, increased consumption, decreased production, increased sequestration, and immune-mediated destruction of platelets) alone or in combination make thrombocytopenia very common in intensive care unit (ICU) patients. Persisting thrombocytopenia in critically ill patients is associated with, but not causative of, increased mortality. Identification of the underlying cause is key for management decisions in individual patients. While platelet transfusion might be indicated in patients with impaired platelet production or increased platelet destruction, it could be deleterious in patients with increased intravascular platelet activation. Sepsis and trauma are the most common causes of thrombocytopenia in the ICU. In these patients, treatment of the underlying disease will also increase platelet counts. Heparin-induced thrombocytopenia requires alternative anticoagulation at a therapeutic dose and immune thrombocytopenia immunomodulatory treatment. Thrombocytopenia with symptomatic bleeding at or above World Health Organization grade 2 or planned invasive procedures are established indications for platelet transfusions, while the evidence for a benefit of prophylactic platelet transfusions is weak and controversial. If the platelet count does not increase after transfusion of 2 fresh ABO blood group–identical platelet concentrates (therapeutic units), ongoing platelet consumption and high-titer anti-HLA class I antibodies should be considered. The latter requires transfusion of HLA-compatible platelet concentrates.
Introduction
Thrombocytopenia is generally defined as platelet counts <150 × 109/L and represents a common laboratory finding in intensive care unit (ICU) patients. Up to 50% of patients present with thrombocytopenia at some time point of their ICU stay, and 5% to 20% develop severe thrombocytopenia, defined as platelet counts <50 × 109/L.1-3 Normally, the platelet count in the peripheral blood is controlled by complex interactions regulating platelet production in the bone marrow, platelet pooling in the liver and spleen, and their elimination in the reticuloendothelial system,4,5 which then feeds back into thrombopoietin regulation.6 The platelet count is rather constant in an individual person.7 In critically ill patients, however, these mechanisms can fail, which results in a disturbance of the balance among platelet production, platelet pooling, and platelet consumption. Thrombocytopenia should be seen as a sensitive marker for considerable alteration of normal physiology. This is most likely the reason for the consistent finding that a low platelet count is associated with an increased risk of mortality in critically ill patients.8-15 For example, in a prospective observational study that analyzed 257 patients who stayed >2 weeks in the ICU, 138 patients (54%) presented with thrombocytopenia of <150 × 109/L on day 4 after ICU admission. They had a higher mortality than nonthrombocytopenic patients (33% vs 16%; P < .05).8 While in most patients the platelet count returned to normal in the second week, for 51 of the 257 patients, thrombocytopenia remained on day 14; these patients had the highest mortality (66% vs 16%; P < .05).8
The mechanisms contributing to thrombocytopenia in the ICU include the following: (1) pseudothrombocytopenia, (2) hemodilution, (3) platelet consumption, (4) decreased platelet production, (5) increased sequestration of platelets, and (6) immune-mediated destruction of platelets.16 In the individual thrombocytopenic ICU patient, often more than one of these mechanisms is responsible for the low platelet count; for example, thrombocytopenia in sepsis results from decreased production as well as increased consumption and destruction of platelets17,18 (eg, by hemolysins).19 Table 1 correlates these mechanisms with potential underlying diseases in typical ICU patients.
A crucial step for the successful treatment of thrombocytopenia in the critically ill is to identify the underlying cause(s) of the low platelet count, as management differs substantially depending on the underlying disease. While platelet transfusion might be indicated in patients with impaired platelet production or increased platelet consumption or destruction, it could be deleterious in patients with increased intravascular platelet activation, like in heparin-induced thrombocytopenia (HIT) or TTP20,21 and possibly in certain prothrombotic forms of disseminated intravascular coagulopathy. In the following, using illustrative patient cases, we discuss the different causes of thrombocytopenia and the implications for patient management.
As with any complex disease, a detailed history and careful physical examination are key to achieving the right diagnosis. Supported by a few laboratory tests, interpretation of these data within the specific clinical context often enables diagnosis.
In cases of unexpected thrombocytopenia, the first question should be whether the patient is really thrombocytopenic.
Case 1: pseudothrombocytopenia
Scenario
A 67-year-old male patient with acute coronary syndrome received emergency percutaneous coronary intervention with implantation of several stents, one of them in the left main coronary artery, followed by therapeutic dose anticoagulation with unfractionated heparin (UFH) plus platelet inhibition with aspirin, clopidogrel, and eptifibatide (all in standard doses). Six hours postintervention, the platelet count had dropped from 270 × 109/L (preprocedure) to 6 × 109/L (in EDTA-anticoagulated blood as well as in citrated blood), and the patient was admitted to the ICU due to the anticipated risk of major bleeding, although he did not show overt bleeding symptoms.
Management
The case raises several management issues. Should all antiplatelet drugs, including eptifibatide, be stopped? Should heparin be stopped? Should platelets be transfused to prevent bleeding? Should tranexamic acid be given prophylactically? As physical examination revealed no bleeding signs, immediate review of the blood film was requested, which showed large platelet aggregates, confirming the diagnosis of eptifibatide-induced pseudothrombocytopenia. The patient was transferred back to the cardiology ward for ongoing standard poststenting treatment.
Comment
Pseudothrombocytopenia is a laboratory artifact usually caused by in vitro platelet agglutination in EDTA-anticoagulated blood. These platelet aggregates cannot be recognized by automated cell counters, thus underestimating the true platelet count. Naturally occurring immunoglobulin class M antibodies directed against epitopes on platelet glycoprotein IIbIIIa (GPIIbIIIa), which are expressed upon calcium chelation by EDTA,22,23 cause in vitro platelet clumping and spurious thrombocytopenia. In most cases, the diagnosis is confirmed by measuring normal platelet counts in citrated blood. However, during treatment with GPIIbIIIa antagonists, pseudothrombocytopenia can also occur in citrated blood.24
As GPIIbIIIa antagonists can induce both real thrombocytopenia and pseudothrombocytopenia in >3% of patients,25,26 exclusion of pseudothrombocytopenia is crucial before any antiplatelet therapy is stopped or prothrombotic treatments are even initiated due to the high risk of acute in-stent thrombosis.27 Also of note, in cases of real GPIIbIIIa-inhibitor–induced thrombocytopenia major bleeding complications are rare, and cessation of the GPIIbIIIa inhibitor is usually sufficient.
Case 2: sepsis
Scenario
A confused 64-year-old male patient with a history of multiple myeloma who had received a cycle of antineoplastic therapy (bortezomib and dexamethasone) the previous day was admitted to the ICU with septic fever, shivering, and circulatory shock. A several-day-old blunt trauma of the right forearm was seen during physical examination. Laboratory assessment indicated massive systemic inflammation (C-reactive protein, 247.0 mg/L [normal value ≤5.0 mg/L]; procalcitonin, 223 ng/mL [0-0.5 ng/mL]); renal failure [creatinine, 392 µmol/L (49-97 µmol/L)]; and coagulopathy (international normalized ratio, 1.7; activated partial thromboplastin time [aPTT], 34 s [25-33 s]). The platelet count was 48 × 109/L (Figure 1A).
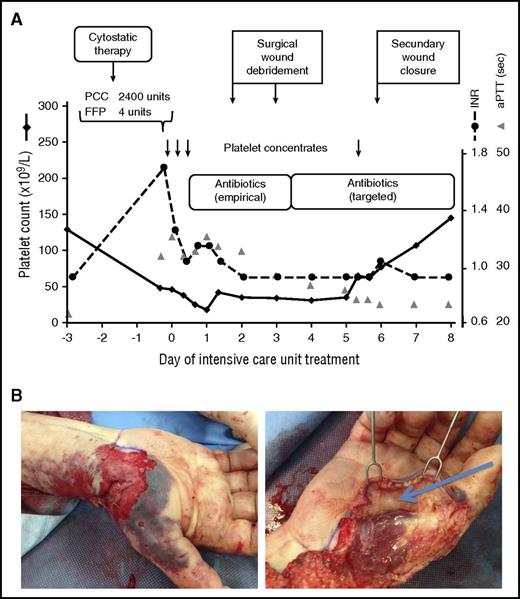
Thrombocytopenia in a septic ICU patient. Although recent cytostatic therapy could have been the reason of the low platelet count at ICU admission (48 × 109/L), sepsis from an infected lower arm wound was responsible for the low platelet count. Causative therapy of sepsis, and thereby of thrombocytopenia, consisted of antibiotic therapy and surgical source control. Transfusion of fresh frozen plasma (FFP), prothrombin complex (PCC), and 4 therapeutic platelet concentrates (each containing 2-4 × 1011 platelets per unit) were given to allow the surgical treatment. (Four-factor PCCs are used in some European centers to improve hemostasis, especially if the patient does not tolerate transfusion of large fluid volumes. This differs from current medical practice in North America.) After the source of sepsis could have been controlled, the platelet count returned to normal without any further measures (A). INR, international normalized ratio; sec, seconds. (B) Operative site during debridement of the infected right forearm injury at an earlier (top) and a later (bottom) time point of surgery.
Thrombocytopenia in a septic ICU patient. Although recent cytostatic therapy could have been the reason of the low platelet count at ICU admission (48 × 109/L), sepsis from an infected lower arm wound was responsible for the low platelet count. Causative therapy of sepsis, and thereby of thrombocytopenia, consisted of antibiotic therapy and surgical source control. Transfusion of fresh frozen plasma (FFP), prothrombin complex (PCC), and 4 therapeutic platelet concentrates (each containing 2-4 × 1011 platelets per unit) were given to allow the surgical treatment. (Four-factor PCCs are used in some European centers to improve hemostasis, especially if the patient does not tolerate transfusion of large fluid volumes. This differs from current medical practice in North America.) After the source of sepsis could have been controlled, the platelet count returned to normal without any further measures (A). INR, international normalized ratio; sec, seconds. (B) Operative site during debridement of the infected right forearm injury at an earlier (top) and a later (bottom) time point of surgery.
Management
The low platelet count could have been associated with multiple myeloma and/or antineoplastic therapy. However, considering the clinical context, the most likely reason was sepsis with an unknown focus. Further workup excluded the most frequent causes of sepsis: pneumonia (chest x-ray), urinary tract infections (urine stick), or peritonitis (sonography and physical examination). Infection of the wound at the right forearm injury was then assumed as the source of sepsis in this immunocompromised patient. After taking blood cultures and starting broad-spectrum antibiotics, surgical debridement was performed (Figure 1B), for which 3 therapeutic platelet concentrates (each containing 2-4 × 1011 platelets per unit) were given prophylactically. The second prophylactic platelet transfusion was given 5 days later to allow surgical closure of the forearm wound. Subsequently, the platelet count normalized as an indicator of successful sepsis treatment (Figure 1A).
Comment
This case illustrates how important it is to identify and control the source of sepsis to allow normalization of the coagulation system, including the platelet count. Sepsis accounts for ∼50% of all thrombocytopenias in the severely ill.17 The mechanisms leading to thrombocytopenia associated with sepsis are multifactorial and complex and include decreased platelet production, increased platelet consumption, and sequestration, frequently by hemophagocytosis.12 Enhanced platelet consumption results from ongoing thrombin generation and increased adhesion of platelets to endothelial cells.17,28 In critically ill septic patients, thrombocytopenia is associated with a dysregulated host response,29,30 and it indicates poor prognosis in patients with septic shock.31
Therapy of sepsis32 requires source control, antibiotic therapy, and supportive measures. Platelet transfusion is recommended in case of bleeding at or above World Health Organization grade 2 (ie, more than mild blood loss like epistaxis, hematuria, hematemesis),33 but invasive interventions like debridement of infected tissue may require prophylactic platelet transfusions.33
Case 3: trauma
Scenario
An 18-year-old female patient with multiple trauma after a fall from the fourth floor, including subarachnoidal hemorrhage, bilateral hemopneumothorax, and pelvic fracture, was admitted with hemorrhagic shock requiring prehospital resuscitation. Despite severe anemia (hemoglobin, 6.0 g/dL) and coagulopathy (international normalized ratio, 1.3; aPTT, 48 s), the platelet count was normal (299 × 109/L) at admission. Computed tomography scan revealed retroperitoneal bleeding and ruptured pelvic vessels. Despite transfusion of 2 platelet concentrates as part of the massive transfusion protocol, the platelet count rapidly declined during the first 7 hours after admission to 51 × 109/L (Figure 2).
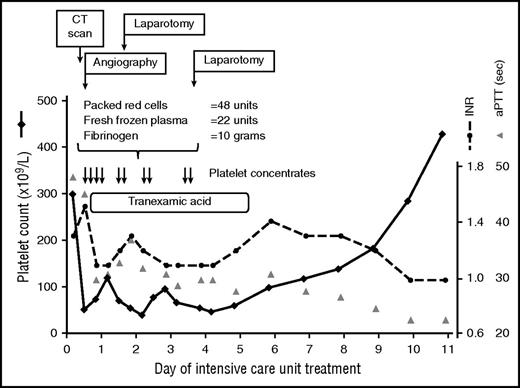
Thrombocytopenia in an ICU patient with multiple trauma. Transfusion of platelet concentrates (each containing 2-4 × 1011 platelets per unit) was part of the massive transfusion protocol. The platelet count returned to normal after the source of bleeding was controlled using surgical and interventional measures. CT, computed tomography; INR, international normalized ratio; sec, seconds.
Thrombocytopenia in an ICU patient with multiple trauma. Transfusion of platelet concentrates (each containing 2-4 × 1011 platelets per unit) was part of the massive transfusion protocol. The platelet count returned to normal after the source of bleeding was controlled using surgical and interventional measures. CT, computed tomography; INR, international normalized ratio; sec, seconds.
Management
Traumatic bleeding from the pelvic arteries prompted embolization of the bleeding artery and subsequent retroperitoneal packing to control hemorrhage. In addition to massive transfusion (48 U red blood cells and 22 U fresh frozen plasma [FFP]), fibrinogen was administered, as fibrinogen is the first coagulation factor that falls below critical values in the case of major bleeding34 (Figure 2). During the first 4 days, this patient was transfused with a total of 10 platelet concentrates (each 2-4 × 1011 platelets per unit), mostly during (or shortly before) invasive procedures, with a targeted platelet count of >50 × 109/L. The platelet count increased spontaneously after bleeding was stopped by interventional and surgical procedures (Figure 2).
Comment
Trauma-induced coagulopathy together with hemodilution due to massive transfusion of red blood cells and FFPs35 is a common cause of thrombocytopenia in the ICU.17 In the presented case, severe trauma, massive bleeding (a more than twofold loss of blood volume),34 and coagulopathy led to trauma-induced platelet loss and consumption. In addition, consumption of plasmatic coagulation factors, hyperfibrinolysis, and systemic inflammation36 as well as hemodilution due to infusion of fluids and transfusion of blood products, and shock-related metabolic acidosis enhance the bleeding risk.37
In actively bleeding trauma patients, transfusion of platelet concentrates alone will usually not stop bleeding, but this situation of consumptive coagulopathy is nevertheless an absolute indication for platelet transfusion as a bridging therapy to maintain the target platelet count until the surgeon or the interventionalist mechanically stops the bleeding. Current guidelines recommend platelet transfusions to maintain the platelet count >50 × 109/L38 in trauma patients and >100 × 109/L in patients with ongoing bleeding and/or traumatic brain injury.36 In addition, tranexamic acid was given to correct hyperfibrinolysis.36,39,40 In the CRASH-2 trial41 application of 1 g tranexamic acid as a bolus followed by infusion of another 1 g over 8 hours reduced mortality in adult trauma patients compared with placebo (4.9% vs 5.7%; relative risk 0.85, 95% confidence interval 0.76-0.96; P = .0077). Tranexamic acid should be given as early as possible in trauma patients.42
Case 4: ITP
Scenario
A 46-year-old male patient with a 10-year history of ITP suffered traumatic brain contusion from a bicycle accident. At ICU admission, the platelet count was 11 × 109/L, and the neurosurgeons required 50 × 109/L platelets in order to control bleeding. The outpatient file of this patient documented previous good platelet count responses to corticosteroids and IVIG.
Management
We transfused 4 therapeutic units of platelet concentrates targeting a platelet count of > 35 × 109/L at admission and gave 1 g IVIG per kilogram body weight per day for 2 days, together with 100 mg prednisone during the first week. Platelet-inhibiting agents (eg, nonsteroidal anti-inflammatory drugs) were avoided, and no routine heparin thromboprophylaxis was given. Repeated computed tomography scans over the next few days showed the brain contusions at a constant size, and the patient remained in stable clinical condition.
However, on day 5, he developed a symptomatic pulmonary embolism. At that time, the platelet count was 60 × 109/L. UFH was given in escalating doses, first achieving an aPTT of 40 s and later 50 s. The cardiopulmonary condition stabilized, and the neurologic state remained stable.
Comment
Onset of ITP is extremely rare in adult ICU patients, although the incidence of ITP (3-5/100 000 population)43 makes it possible that a patient with chronic ITP is admitted to the ICU with life-threatening bleeding. Because ITP is an autoimmune disorder characterized by immunologic destruction of otherwise normal platelets,44 immunosuppressive therapy is the first-line treatment. The administration of 2 g IVIG per kilogram body weight (over 2 consecutive days), followed by 100 mg prednisone per day has been shown to result in the fastest increase of the platelet count in adult patients with (untreated) severe ITP.45 In contrast, platelet transfusions are usually not effective, but transfusion of large amounts of platelets can result in cessation of bleeding and even an increased platelet count and are therefore recommended as first-line treatment (in addition to IVIG and corticosteroids) in ITP patients with life-threatening bleeding.44,46,47 However, in this situation, often more than 5 therapeutic units are required before bleeding is controlled.
Severe thrombocytopenia, especially if associated with symptomatic bleeding, is a well-established contraindication for treatment with an anticoagulant, including pharmacological thrombosis prophylaxis.48 In the absence of prospective randomized trials, a widely used protocol in patients with malignancy-associated thrombocytopenia who have comorbidities, but who typically require therapeutic-dose anticoagulation (eg, for the treatment of symptomatic venous thromboembolism), is to reduce therapeutic-dose anticoagulation by half if the platelet count falls below 50 × 109/L, to use prophylactic dose anticoagulation if the platelet count falls below 30 × 109/L, and to stop all anticoagulants if the platelet count falls below 20 × 109/L.49,50 Patients with ITP have an increased risk of thrombosis,51 as do patients with intracranial hemorrhage undergoing neurosurgery.52 In these patients, thrombocytopenia per se does not necessarily protect against thrombosis in otherwise high-risk situations. In the absence of acute bleeding, and especially when the platelet count has responded to treatment, increasing above 20 × 109/L, critically ill ITP patients with additional risk factors for thrombosis should receive pharmacologic thromboprophylaxis to reduce the risk of acute thrombosis and pulmonary embolism, unless there is overt bleeding. Acute thrombosis mandates therapeutic-dose anticoagulation for the next 3 months, substantially increasing the risk of major bleeding in patients with chronic ITP unless the platelet count can be stabilized at a higher level during this period (eg, by thrombopoietin receptor agonists).53-55
Case 5: drug-induced thrombocytopenia
Scenario
A 75-year-old female patient with stroke and right-sided hemiplegia, recurrent seizures, dysphagia, and pneumonia required invasive ventilation. She received several drugs, including antibiotics, sedation, aspirin, UFH in prophylactic dose, diuretics, and anticonvulsants. At day 7, valproic acid was added to levetiracetam and lorazepam to control seizures. Ten days later, the platelet count began to fall, reaching values <50 × 109/L (Figure 3).
Stopping the valproate medication was sufficient to treat thrombocytopenia in a case of valproate-induced DTP.
Stopping the valproate medication was sufficient to treat thrombocytopenia in a case of valproate-induced DTP.
Management
In the absence of any laboratory or clinical symptoms for sepsis, HIT was first suspected in our patient. When we applied the 4Ts score56 (Table 2), it revealed 4 points (platelet count decrease >50% = 2 points; onset of platelet count fall >day 10 = 1 point; no thrombosis = 0 points; thrombocytopenia in a patient on a ventilator as an assumed other reason for thrombocytopenia = 1 point), consistent with an intermediate risk for HIT, but the anti–platelet factor 4 (anti-PPF4)/heparin immunoglobulin G (IgG) enzyme immunoassay was negative, thus ruling out HIT.57,58
Comment
Drug-related thrombocytopenia is a relatively common cause of thrombocytopenia in ICU patients,17 whereby drug-induced nonimmune thrombocytopenia (DTP) (eg, that caused by toxic bone marrow suppression) is responsible for the vast majority of cases.60-65 With the exception of HIT, drug-induced immune thrombocytopenia (DITP) is much less frequent than DTP.17,60,64 In contrast to DTP, DITP typically presents with an abrupt platelet count fall evolving within 1 to 2 days, which usually begins 5 to 14 days after starting a new drug, and a nadir below 20 × 109/L, which is nearly always accompanied by mucocutaneous bleeding.59,65-69 Table 3 summarizes typical mechanisms and drugs of DTP and DITP.61-63,65,70
More than 10% of patients treated with valproic acid develop DTP.59 Older age, female sex, higher valproic acid dosage, and lower baseline platelet counts are associated with an increased risk of DTP.59,66,67 However, valproic acid can also induce immune-mediated DITP. In both DTP and DITP, cessation of the drug is most important and usually sufficient.59,65-69 Recovery of the platelet count will occur thereafter and is often not very helpful for differentiation between DTP and DITP. In our patient, the platelet count started to increase ∼48 hours after stopping valproic acid, but also in DITP, platelet count recovery usually begins 5 to 7 half-times after cessation of the drug (half-time for valproic acid 5-7 hours = expected recovery after ∼50 hours). The relatively slow decline of the platelet count and especially the nadir above 20 × 109/L argued against DITP.
If a patient with DITP develops major bleeding symptoms, IVIG (1 g/kg body weight on 2 consecutive days) is recommended.65 This has augmented platelet count recovery in mouse models of DITP.71 Corticosteroids, however, are usually ineffective.65 In case of life-threatening bleeding, transfusion of platelet concentrates might be considered.
Laboratory tests for the detection of drug-dependent antiplatelet antibodies are helpful to support the diagnosis DITP.63 However, these tests are performed only in specialized laboratories and are usually not available to guide acute management.72 In contrast to assays detecting antibodies in HIT, the sensitivity of assays for all other DITPs is low, and thus a negative test result does not rule out the diagnosis. The clinical relevance of laboratory testing for DITP antibodies is twofold. First, it allows objective confirmation of an adverse drug effect (relevant for pharmacovigilance). Second, it is important for the individual patient (future drug avoidance). We could not demonstrate valproic acid–dependent platelet-reactive antibodies in this patient, further supporting nonimmune mechanisms.
Case 6: heparin-induced thrombocytopenia
Scenario
A 79-year-old female patient on aspirin and clopidogrel after implantation of coronary stents several weeks earlier developed in-stent thrombosis with myocardial infarction and cardiac arrest. After successful cardiopulmonary resuscitation and coronary angioplasty, the patient was admitted to the ICU. Aspirin and clopidogrel were continued, and therapeutic-dose UFH was started. The patient was in cardiogenic shock with multiple organ failure, and the platelet count dropped from 326 to 28 × 109/L by day 5 of ICU treatment (Figure 4).
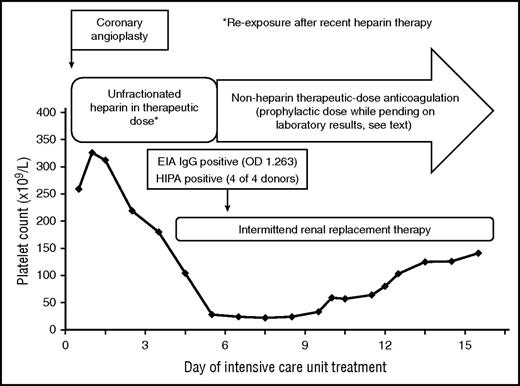
HIT was assumed to be the cause of thrombocytopenia beyond day 5 of ICU treatment, because high-titer antiplatelet factor 4 immunoglobulin G antibodies were detectable by enzyme-immunoassay and the heparin-induced activation assay was strongly positive at day 6 of heparin treatment. However, the moderate increase of the platelet count after switching to nonheparins indicated that HIT was probably not the only cause of thrombocytopenia. EIA, enzyme-immunoassay; HIPA, heparin-induced platelet activation assay; OD, optical density.
HIT was assumed to be the cause of thrombocytopenia beyond day 5 of ICU treatment, because high-titer antiplatelet factor 4 immunoglobulin G antibodies were detectable by enzyme-immunoassay and the heparin-induced activation assay was strongly positive at day 6 of heparin treatment. However, the moderate increase of the platelet count after switching to nonheparins indicated that HIT was probably not the only cause of thrombocytopenia. EIA, enzyme-immunoassay; HIPA, heparin-induced platelet activation assay; OD, optical density.
Comment
Analyzing carefully the platelet count course is helpful for discerning among various explanations for thrombocytopenia. Typically, ICU patients present with a biphasic platelet count course. After an initial decrease to a platelet count nadir 2 to 4 days after ICU admission, platelets recover to higher-than-baseline values (so-called reactive thrombocytosis).8 Persistent or progressive thrombocytopenia therefore suggests ongoing consumption, bleeding, or severe organ damage. A slow decrease in platelet counts over several days is rather typical for infection, septicemia, or bone marrow toxicity. Immune-mediated causes, such as HIT or DITP,73 should be considered when the platelet count falls rapidly within 1 or 2 days during the second week of treatment after an initial recovery.
Management
The initial platelet count decrease was easily explained by severe disease. However, when the platelets fell to <30 × 109/L at day 5 we included HIT into the differential diagnoses of the low platelet count. The 4Ts score56 was 4 points (2 points for the thrombocytopenia, 1 point for the timing, 0 points for thromboses, and 1 point for other reasons), giving an intermediate pretest probability of HIT. As the probability of HIT is >25% in patients with a 4Ts score of ≥4,56 we introduced alternative anticoagulation using danaparoid in prophylactic dose. Because the suspicion of HIT was vague and the risk of bleeding high, we first used prophylactic dose danaparoid to avoid further triggering HIT without increasing the bleeding risk. After the HIT antibody tests were highly suggestive for HIT (strongly positive anti-PF4/heparin IgG antibodies in the enzyme immunoassay and detection of heparin-dependent platelet-activating antibodies in the heparin-induced platelet activation assay; Figure 4), we escalated to therapeutic-dose danaparoid. The positive HIT-antibody test results made the diagnosis of HIT possible (although HIT may not necessarily be present in the setting of early-onset and persisting thrombocytopenia, even when HIT test results are positive).74 Indeed, the platelet count increased only moderately (Figure 4) over the next 7 days, indicating that factors other than HIT were probably most responsible for thrombocytopenia.
Comment
Even in hindsight, it is unclear whether the strong platelet-activating anti-PF4/heparin antibodies were really the cause of the low platelet count or simply an epiphenomenon, as these antibodies are detected incidentally in some patients without thrombocytopenia, especially after cardiac surgery (this may be the case in >10% of patients).74,75 However, the further worsening of thrombocytopenia 5 days after starting heparin, at a time when platelet count recovery would otherwise have been expected, together with a strongly positive functional assay, prompted us to change anticoagulation to a nonheparin anticoagulant, given the risk for new thrombosis (up to 5% per day) in acute HIT,76 in the absence of an effective anticoagulant.
HIT is immune mediated and usually occurs 5 to 14 days after starting heparin in case of HIT. However, in contrast to other DITP, (1) platelets fall below 20 × 109/L in only ∼10 to 15% of cases, (2) thromboses rather than bleeding are the typical clinical complications, (3) treatment requires substituting heparin with an alternative (nonheparin) anticoagulant, and (4) antibodies usually disappear 50 to 80 days after the acute episode of HIT. This makes re-exposure to heparin possible under special circumstances, for example, during cardiovascular surgery, when antibodies are no longer detectable.77 HIT can occur within hours in preimmunized patients who receive heparin when platelet-activating anti-PF4/heparin antibodies are still present in the circulation (so-called rapid-onset HIT). Although this had been possible in our patient due to pretreatment with heparin during the first episode of acute coronary syndrome a few weeks before, rapid-onset HIT appeared unlikely, as the platelet count started to decrease after day 2 only.
Platelet transfusions in ICU patients
A major issue in thrombocytopenic ICU patients is whether and when platelet transfusions should be given:
Therapeutic platelet transfusions are generally indicated in patients with bleeding at or above World Health Organization grade 2 (ie, more than mild blood loss like epistaxis, hematuria, hematemesis).33,78 In many ICU patients, thrombocytopenia is associated with acquired mild to moderate platelet function defects,79-81 which are caused or aggravated by medications such as antibiotics and 82,83 analgesics, platelet activation on extracorporeal circuits,84 or cleavage of platelet receptors by released enzymes in cases of sepsis.28 Therefore, in thrombocytopenic patients, bleeding symptoms are much more relevant than the platelet count when deciding whether platelets should be transfused. In patients with immune-mediated thrombocytopenia (eg, ITP and DITP), however, platelet transfusions should be restricted to patients with serious or life-threatening bleeding.47,65 The same accounts for patients with acute HIT or TTP.85,86
Due to the lack of prospective randomized trials, major uncertainty exists in regard to prophylactic platelet transfusions in ICU patients with thrombocytopenia (with or without disseminated intravascular coagulopathy) and/or platelet function defects without active bleeding at or above WHO grade 2. As in patients with hypoproliferative thrombocytopenia due to chemotherapy, ICU patients with spontaneous bleeding of the oropharyngeal mucous membranes (so-called wet purpura) are at increased risk of bleeding into the central nervous system or retinal bleeding and should receive prompt platelet transfusions in case of non–immune-mediated thrombocytopenia. In patients with immune-mediated thrombocytopenia (eg, ITP and DITP), however, other measures such as high-dose IVIG are more appropriate. As no data exist demonstrating that ICU patients benefit from prophylactic platelet transfusion in regard to bleeding or mortality,87 we consider it reasonable to restrict platelet transfusions to ICU patients with symptomatic bleeding irrespective of the platelet count. However, a recent expert recommendation suggests prophylactic transfusion of platelets in patients with severe sepsis at a threshold of ≤10 × 109/L, and if the patient has a significant risk of bleeding, a threshold of ≤20 × 109/L is recommended.32
Another issue is platelet transfusion before invasive procedures for which only observational studies exist. Table 4 summarizes the established recommendations for prophylactic platelet transfusions before invasive procedures and the strength of the recommendation.33,88
A further dilemma in critically ill patients is the evaluation of whether a platelet transfusion was successful. Often one cannot observe the expected platelet count increase of ∼15 × 109/L in an ICU patient after transfusion of a single therapeutic platelet unit,87 because ongoing consumption of platelets due to the underlying disease or concomitant treatment (eg, during application of antimycotic drugs). Transfusion of 2 fresh ABO blood group–identical platelet concentrates (therapeutic units) usually overcomes nonimmune causes of platelet transfusion refractoriness. If the platelet count still does not increase, the presence of platelet-reactive antibodies, especially anti-HLA class I antibodies, should be excluded, especially in multiparous women. If high-titer anti-HLA class I antibodies are detectable, HLA compatible platelet concentrates often result in an appropriate platelet count increase. Rarely, anti–human platelet antigen alloantibodies are implicated in patients who test positive for anti-HLA antibodies but remain refractory to HLA-matched platelet concentrates.89
Summary
Thrombocytopenia is common in the ICU. It is a sensitive marker for the severity of the disease and associated with increased mortality. Identifying the underlying cause is essential for successful treatment. Platelet transfusions can be helpful in situations of platelet loss and/or consumption, but they might be deleterious in patients with increased intravascular platelet activation. A detailed history and careful physical examination are keys to achieving the right diagnosis, supported by a few laboratory test results and interpreting these data within the clinical context.
Authorship
Contribution: A.G. and S.S. contributed equally to the manuscript.
Conflict-of-interest disclosure: S.S. and A.G. are members of the current American Society of Hematology guideline committee on heparin-induced thrombocytopenia. A.G. is a member of the International Society on Thrombosis and Hemostasis scientific subcommittee on platelet immunology, cochair of the subcommittee on perioperative management, a member of the working group “Guidelines for treatment with blood components of the German Medical Association – der Bundesärztekammer,” a member of the regular working group “Guidelines for production of blood and blood components and the treatment with blood products” (Hemotherapy) der Bundesärztekammer.” S.S. declares no competing financial interests.
Correspondence: Andreas Greinacher, Institute of Immunology and Transfusion Medicine, University Medicine Greifswald, Sauerbruchstraße, 17495 Greifswald, Germany; e-mail: greinach@uni-greifswald.de.
References
Author notes
A.G. and S.S. contributed equally to this study.