Key Points
A novel PTP1B variant, PTP1BΔ6, is expressed in cHL cell lines and tumor samples.
PTP1BΔ6 augments JAK/STAT activity, cell proliferation, and survival in cHL cell lines.
Abstract
Chronic activation of the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling pathways is a hallmark of a variety of B-cell lymphomas, including classical Hodgkin lymphoma (cHL). Constitutive JAK/STAT signaling is crucial for survival and proliferation of Hodgkin/Reed-Sternberg (HRS) cells, the malignant cells of cHL. Although the molecular basis of this constitutive JAK/STAT signaling in cHL has not been completely understood, accumulating reports highlight the role of an inactivation or reduced expression of negative JAK/STAT regulators such as silencer of cell signaling 1 (SOCS1) or protein-tyrosine phosphatase 1B (PTP1B) in this process. Here, we report the expression of truncated PTP1B mRNA variants identified in cHL cell lines and primary cHL tumor samples lacking either 1 or several exon sequences. One of these novel PTP1B variants, a splice variant lacking exon 6 (PTP1BΔ6), was found expressed at low levels in cHL cell lines. However, serum stimulation of cHL augmented the expression of PTP1BΔ6 significantly. Functional characterization of PTP1BΔ6 revealed a positive effect on interferon-γ- and interleukin-4-induced JAK/STAT activity in HEK293 or HEK293-STAT6 cells, and on the basal STAT activity in stably transfected L-428 and U-HO1 cHL cell lines. Furthermore, PTP1BΔ6 expression increased the proliferation of L-428 and U-HO1 cells and reduced cytotoxic effects of the chemotherapeutical agents gemcitabine and etoposide distinctively. Collectively, these data indicate that PTP1BΔ6 is a positive regulator of JAK/STAT signaling in cHL.
Introduction
Signal transducer and activator of transcription (STAT) molecules comprise a family of transcription factors involved in the control of cell survival, differentiation, and proliferation (reviewed in Abroun et al1 and O’Shea et al2 ). Activation of the STAT transcription factors by cytokines or growth factors involves the recruitment and activation of members of the Janus kinase (JAK) family, JAK1, JAK2, JAK3, and TYK2. Activated JAKs, in turn, phosphorylate STAT proteins at specific tyrosine residues, triggering dimerization, nuclear translocation, and binding of the STAT proteins to their cognate sites in the promoter regions of a variety of target genes involved in cell cycle control (eg, cyclin D1, c-myc, p21) and cell survival (eg, BCLXL, MCL1, BCL2).
Constitutive activation of the JAK/STAT pathway is essential for a variety of B-cell lymphomas, including diffuse large B-cell lymphoma (DLBCL), primary mediastinal B-cell lymphoma (PMBL), and classical Hodgkin lymphoma (cHL).3,4 Recurrent inactivating mutations in SOCS1, protein tyrosine phosphatase N1 (PTPN1), and protein tyrosine phosphatase N2 (PTPN2) encoding negative JAK/STAT regulators appear to participate in the constitutive JAK/STAT activation in DLBCL, PMBL, and cHL.5-9 The cHL cell line U-HO1, for instance, harbors a biallelic mutation of PTPN1, whereas additional PTPN1 point mutations including nonsense, missense, or frame-shift mutations were identified in samples from patients with PMBL and cHL.5,9,10 Several of these mutants display a diminished phosphatase activity and are less capable of reducing STAT activity in interleukin 4 (IL-4)-stimulated cells.
The PTPN1 product protein-tyrosine phosphatase 1B (PTP1B) is anchored in the membrane of the endoplasmic reticulum (ER), pointing with its amino-terminal catalytic domain into the cytoplasm, where it dephosphorylates receptor tyrosine kinases, cytokine receptors, JAKs, and STAT proteins, thereby terminating cytokine-induced JAK/STAT signaling.11 PTP1B activity itself is regulated by cytokine-mediated gene induction, site-specific phosphorylation by AKT, oxidation of the cysteine residue in the active site, limited proteolysis, and cytokine-induced alternative splicing.12 This PTPN1 splice variant retains the intron 9, causing a frame shift in the 3′ end leading to a loss of the carboxy-terminal ER membrane anchor. Although the functions of this PTP1BΔER variant remain unknown, it seems possible that this soluble protein might bind to a different set of substrates.
The role of PTP1B in tumorigenesis is variable and depends on the tumor entity. As mentioned before, PTP1B acts as tumor suppressor in B-cell lymphoma. In contrast, for breast and colorectal carcinoma, a pro-oncogenic function of PTP1B has been reported.13-16 In breast carcinoma cell lines, for instance, PTP1B has been shown to activate c-Src by dephosphorylating its negative regulatory residue Tyr530. PTP1B activates the Ras-Raf-ERK oncogenic signaling pathway, most likely by dephosphorylating the scaffold protein p62Dok, an activator of p120RasGAP.
Given the pivotal role of PTP1B for JAK/STAT signaling in conjunction with the importance of this signaling pathway for the pathogenesis of cHL, we aimed at further dissecting the mechanisms controlling PTP1B activity in cHL. In particular, we wanted to determine the expression of alternative PTPN1 mRNA splicing variants. We identified a set of PTPN1 mRNA variants lacking either single or multiple exons from primary cHL cases and cHL cell lines. A PTPN1 variant lacking exon 6, named PTPN1Δ6, encodes for a functional PTP1B protein missing a part of its catalytic domain. PTP1BΔ6 exerted a positive effect on signal-induced STAT activity in HEK293 and HEK293-STAT6 cells. Moreover, in cHL cell lines L-428 and U-HO1, PTP1BΔ6 augmented the constitutive JAK/STAT signaling, leading to enhanced cell proliferation and survival. Taken together, we show that PTP1BΔ6 is a novel positive JAK/STAT regulator in cHL.
Methods
Cell culture, transfection, and lentiviral transduction
Cell culture reagents were from Lonza, fetal calf serum was from Seromed/Biochrom. All cell lines used were grown in IMDM/RPMI (4:1) supplemented with 10% fetal calf serum, glutamine, 100 U/mL penicillin/streptomycin at 37°C, and 5% CO2. HEK293 cells were transfected with the calcium-phosphate method, as described previously.17 Transfection of cHL cell lines using plasmids or siRNA was performed with an Amaxa Cell Line Nucleofector Kit (Lonza). Lentiviral transduction of L-428 and U-HO1 cells was performed by spinoculation, as described.18
Antibodies, reagents, and plasmids
The following antibodies were used: rabbit anti-PTP1B (N-19)-R, mouse anti-PTP1B (D-4), rabbit anti-STAT6 (M-200), rabbit anti-NFκB p65 (C-20) (Santa Cruz), rabbit anti-pStat1 (Tyr701), rabbit anti-Stat1, rabbit anti-pStat6 (Y641) (Cell Signaling), rat anti-HA (Roche), mouse anti-β-Actin and mouse anti-β-tubulin (Sigma). Secondary antibodies were anti-rabbit HRP (Sigma), anti-mouse HRP (Thermo Scientific), and goat anti-rat HRP (Santa Cruz). IL-4 and interferon-γ (IFNγ) were from ImmunoTools. Gemcitabine and etoposide were from Sigma. HA-PTPN1, FLAG-PTPN1, and EXPRESS-PTPN1 vectors were created by inserting the cDNA into the pcDNA3.1 vector in frame behind a HA-tag sequence, in the EcoRI/XbaI sites of pFLAG-CMV2, or in the EcoRI/BamHI sites of pEXPRESS, respectively. The lentiviral expression vector SF-LV-cDNA-EGFP served to generate the lentiviral PTP1B transduction vectors using the AgeI/NotI restriction sites. All cloning was performed using T4 DNA ligase and restriction enzymes from New England Biolabs.
Isolation of mRNA from microdissected Hodgkin/Reed-Sternberg cells and quantitative polymerase chain reaction analysis
The isolation of neoplastic Hodgkin/Reed-Sternberg (HRS) cells or the surrounding non-neoplastic lymphocytes by laser-capture microdissection, the RNA extraction, and the quantitative RNA analysis are described in the supplemental Methods, available on the Blood Web site.
Preparation of protein cell extracts
Whole-cell extracts (WCE) were prepared using TNT buffer (20 mM Tris [tris(hydroxymethyl)aminomethane] at pH 8.0, 200 mM NaCl, 1% Triton-X 100, 1 mM DTT, phosphatase and protease inhibitors) or RIPA buffer (50 mM Tris⋅HCl at pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 1 mM DTT, phosphatase and protease inhibitors). WCE were clarified by centrifugation at 14 000 rpm for 10 min at 4°C. Cytoplasmic and nuclear proteins were extracted by subsequent incubation of cells in hypotonic buffer A (10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid at pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, protease and phosphatase inhibitors) and hypertonic buffer C (20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid at pH 7.9, 0.4 M KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, protease and phosphatase inhibitor).
Luciferase reporter assay
Cells were transfected with different vectors in combination with STAT1 or STAT6 firefly luciferase reporter plasmid and a renilla luciferase reporter plasmid controlled by the human β-actin promoter. Cells were stimulated with either IFNγ (STAT1 reporter) or IL-4 (STAT6 reporter) for 24 hours before lysis using TNT buffer. Luciferase activities were determined using the Dual-Luciferase Reporter Assay System (Promega). Firefly luciferase activity was normalized to the appropriate renilla luciferase values. The experiments were performed in duplicates and were repeated at least 3 times.
Electrophoretic mobility shift assay
Sequences for oligonucleotides used for STAT EMSAs are given in supplemental Table 1. The double-stranded DNA probes were radioactively labeled using 32P-ddCTP and DNA polymerase I. Six micrograms WCE from L-428 and U-HO1 cells were used for EMSA. For supershift analysis, 2 µg of the following antibodies were added: STAT6 (S-20) X, STAT6 (M-200) X, or NF-κB p50 (NLS) X from Santa Cruz. Protein–DNA complexes were separated by gel electrophoresis and visualized by Fuji Medical X-ray film.
Western blot analysis and co-immunoprecipitation
For western blot analysis, 5 to 20 µg protein extract were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (0.2 µm; GE Healthcare), using standard procedures. The membrane was blocked with 5% milk powder in TBS+Tween20 before incubation with primary antibody (1:1000 in TBS+Tween20), subsequently washed and incubated in a TBS-Tween20 solution containing horseradish peroxidase conjugated secondary antibody (1:5000). Detection was performed using ECL-substrates (Pierce). For the co-immunoprecipitation assay, HEK293 cells were transfected with different HA-PTP1B or FLAG-PTP1B encoding constructs. Subsequently, 0.5 to 1 mg of the resulting WCE was mixed with 1 µg antibody. After overnight incubation at 4°C on a rotator, 10 µL of 50% protein G-slurry was added and incubation was continued at 4°C for 1 h. Immunoprecipitates were washed thrice with TNT and once with PBS before separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and western blot analysis.
Proliferation assay
To measure the survival and proliferation of cells, the CellTiter 96 Aqueous One Solution Cell Proliferation Assay (MTS) from Promega was used. Before measuring absorbance at A490 nm, 100 µL cell suspension and 20 µL MTS solution were mixed and incubated for 1.5 hours.
Endpoint nested-polymerase chain reaction
For identification of the different PTPN1 variants, an endpoint nested-polymerase chain reaction (PCR) was performed. For the first PCR round, primer pairs were designed that bind outside of the coding region of PTPN1 and cDNA from either cHL cell lines or isolated HRS cells were used as template. For the second round, 5 µL of the first-round PCR was used together with primers spanning the whole coding region. A Taq-Polymerase Kit from Qiagen was used for all PCR reactions. PCR thermocycling parameters were 95°C for 3 minutes; 40 cycles of 95°C for 1 minute, 53°C for 1 minute, and 72°C for 4 minutes; and an additional step at 72°C for 10 minutes.
Phosphatase assay
For measuring the enzymatic activity of PTP1B, WCE from the stable L-428 cell lines were generated using TNT buffer (without phosphatase inhibitors). Five hundred micrograms WCE in 200 µL TNT buffer was mixed with 0.4 µg/sample PTPN1 antibody (Sigma, HPA012542) or Strepavidin-conjugated sepharose. The samples were incubated on a rotor at 4°C overnight before addition of 20 µL/sample Protein G Sepharose 4 Fast Flow (GE Healthcare) for 1 hour. Subsequently, samples were washed twice in TNT buffer and twice in phosphatase buffer (10 mM Tris at pH 7.5, 50 mM NaCl, 1 mM MnCl2, 2 mM DTT, 10 mM p-nitrophenyl phosphate (PNPP). Finally, 200 µL phosphatase buffer was added per sample and incubated at 37°C at 1400 rpm for 1 to 2 hours. The reaction was stopped by 800 µL 1N NaOH, and the phosphatase activity was determined at an absorbance of 405 nm.
Results
Identification of novel PTP1B variants in cHL
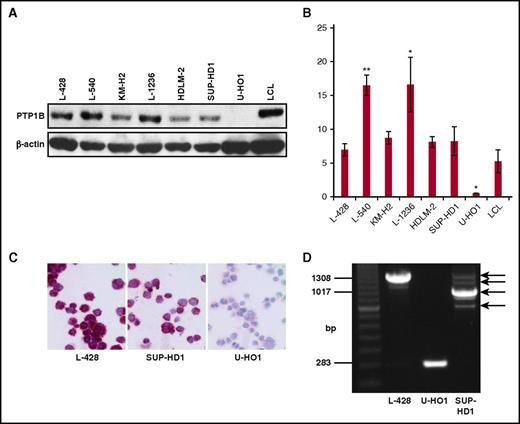
Alternative splicing and proteolytic processing generate altered PTP1B variants with divergent functions und substrates.11,12 Moreover, deletions in PTPN1 of PMBL and cHL tumor samples and cell lines leading to the loss of 1 or more exons have been found.5 The cHL cell line U-HO1 harbors a biallelic mutation with a complete loss of 1 allele and a deletion of exons 2 to 8 in the second PTPN1 allele, whereas SUP-HD1 cells are heterozygous with 1 wild-type (WT) allele and a deletion of exons 2 to 4 in the other PTPN1 allele. As a consequence, PTP1B protein is absent in U-HO1 cells but remains unaffected in SUP-HD1 cells (Figure 1A-C). However, mRNA expression levels and the functionality of the PTP1B variants derived from such deleted PTPN1 genes is unknown. To determine the PTPN1 mRNA profile in Sup-HD1 and U-HO1, an endpoint nested-PCR experiment was performed. Shorter PTPN1 mRNA transcripts were observed in the PTPN1 mutated cHL cell lines U-HO1 (approximately 280 base pairs [bp]) and SUP-HD1 (approximately 900 bp), whereas L-428 cells express an PTPN1 mRNA transcript with the expected length of about 1300 bp. Moreover, additional weaker PTPN1 mRNA signals were observed in L-428 and SUP-HD1 cells (Figure 1D). Thus, shorter PTPN1 transcripts are generated from mutated PTPN1 genes even in presence of a WT allele, as in case of SUP-HD1 cells.
Characterization of PTP1B expression in cHL cell lines. (A) Western blot analysis of the indicated cHL cell lines and a lymphoblastoid cell line (LCL), using PTP1B and β-actin specific antibodies. (B) Quantification of the amount of PTP1B protein as depicted in A. Mean value of 3 independent experiments is shown, and significance was determined by a Student t test in comparison with the LCL values. *P ≤ .05; **P ≤ .01; ***P ≤ .001. (C) Immunocytochemical staining of PTP1B in the cHL cell lines L-428, SUP-HD1, and U-HO1. Original magnification ×400. (D) Endpoint PCR results of the amplified PTP1B cDNA of the cell lines L-428, U-HO1, and SUP-HD1. PCR products corresponding to the PTPN1 cDNAs of different size are marked by arrows.
Characterization of PTP1B expression in cHL cell lines. (A) Western blot analysis of the indicated cHL cell lines and a lymphoblastoid cell line (LCL), using PTP1B and β-actin specific antibodies. (B) Quantification of the amount of PTP1B protein as depicted in A. Mean value of 3 independent experiments is shown, and significance was determined by a Student t test in comparison with the LCL values. *P ≤ .05; **P ≤ .01; ***P ≤ .001. (C) Immunocytochemical staining of PTP1B in the cHL cell lines L-428, SUP-HD1, and U-HO1. Original magnification ×400. (D) Endpoint PCR results of the amplified PTP1B cDNA of the cell lines L-428, U-HO1, and SUP-HD1. PCR products corresponding to the PTPN1 cDNAs of different size are marked by arrows.
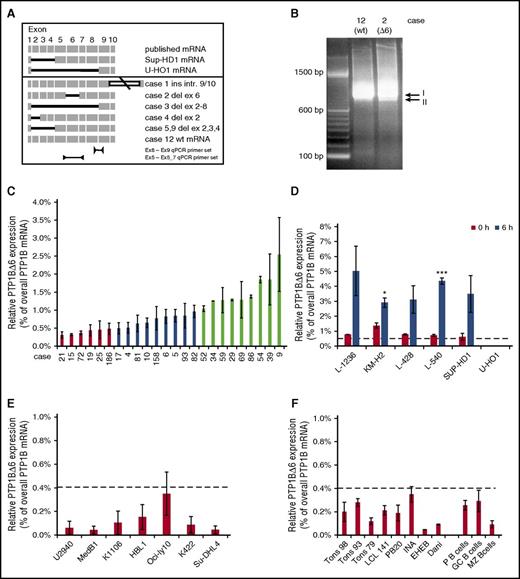
To clarify whether shorter PTPN1 mRNA variants are also expressed in cHL tumor samples, similar endpoint nested-PCR was performed using cDNAs from laser-microdissected HRS cells.7 Indeed, a variety of shorter PTPN1 mRNA variants were detected in cHL tumor samples (see Figure 2A for a schematic representation). Although samples 5 and 9 contained a PTPN1 mRNA lacking exons 2 to 4, similar to SUP-HD1 PTPN1 mRNA, the PTPN1 mRNA from sample 3 resembled U-HO1 PTPN1 mRNA lacking exons 2 to 8. Novel, not-yet-observed PTPN1 mRNA variants were seen in sample 2 (lacking exon 6) and sample 4 (lacking exon 2). In addition, an already-described PTPN1 splice variant with a retaining intron 9 to 10 was found in sample 1. Because PTPN1Δ6 mRNA is the only novel variant coding for a functional yet shorter PTP1B protein, we focused on this variant. First, we determined whether or not PTPN1Δ6 mRNA is a personal variant only seen in this particular cHL sample as a second, faster migrating signal (signal II, Figure 2B). For this, we performed quantitative reverse transcription (qRT) PCR analyses using specific qPCR primer sets for either overall PTPN1 mRNA or the PTPN1Δ6 mRNA (Figure 2A, lower) and determined the relative PTPN1Δ6 mRNA ratio. Relative PTPN1Δ6 expression in laser microdissected HRS cells from cHL tumor samples ranged from 0.4% to 3.5% (Figure 2C), whereas laser-microdissected normal lymphocytes had PTPN1Δ6 levels below 0.3% (supplemental Figure 1). Furthermore, low PTPN1Δ6 mRNA levels in unstimulated cHL cell lines with approximately 1% of total PTPN1 mRNA were found to be increased to approximately 3% to 5% on stimulation with serum for 6 hours (Figure 2D). As these results show that PTPN1Δ6 mRNA expression is not limited to a single cHL case, PTPN1 mRNA profiles from different DLBCL cell lines, LCLs, CD19-positive tonsillar B cells, and complete tonsils were analyzed. As shown in Figure 2D-F, very low levels of PTPN1Δ6 mRNA below 0.4% of the total PTPN1 mRNA pool were also detectable in all analyzed B cells, B lymphoma cells, and tonsils by qPCR. Thus, expression of the PTP1BΔ6 variant is a log level higher in cHL than in all other B-cell systems tested, inviting the question of the function of this novel PTP1B variant in the context of cHL.
Analysis of PTPN1 mRNA variants in cHL. (A) Schematic representation of the identified and sequenced PTPN1 cDNA variants derived from primary cHL cases and cHL cell lines. (B) Endpoint PCR results of the amplified PTPN1 cDNA from laser-microdissected HRS cells of selected primary cHL cases. The PCR signal related to full-length PTPN1 mRNA (I) and the band corresponding to the PTPN1Δ6 mRNA (II) are indicated. (C) Percentage of the PTPN1Δ6 mRNA based on the whole PTPN1 mRNA levels in 24 cases from the cHL cohort measured by qPCR analyses. Three different groups are defined: group I, very low relative PTPN1Δ6 mRNA expression (cases 15, 19, 21, 25, 72, 186; red bars); group II, low relative PTPN1Δ6 mRNA expression (cases 4, 5, 6, 10, 17, 81, 82, 83, 158; blue bars); and group III, high relative PTPN1Δ6 mRNA expression (cases 9, 29, 34, 39, 52, 54, 59, 69, 86; light green bars). (D) Percentage of the PTPN1Δ6 mRNA based on the whole PTPN1 mRNA levels in the different cHL cell lines without and after stimulation with serum for 6 hours, measured by qPCR analyses. Significances are calculated in comparison with the control (0 hours) values. *P ≤ .05; **P ≤ .01; ***P ≤ .001. (E) Percentage of the PTPN1Δ6 mRNA based on the whole PTPN1 mRNA levels in other lymphoma cell lines (PMBL, ABC-DLBCL, GC-DLBCL) measured by qPCR analyses. (F) Percentage of the PTPN1Δ6 mRNA based on the whole PTPN1 mRNA levels in 8 control LCL cell lines (left) and microdissected B cells from lymph node sections. GC, germinal center; MZ, mantle zone (right part). (D-F) The dashed line marks the relative PTPN1Δ6 mRNA expression level of 0.4% observed in the group of laser-captured HRS cells with very low relative PTPN1Δ6 mRNA expression.
Analysis of PTPN1 mRNA variants in cHL. (A) Schematic representation of the identified and sequenced PTPN1 cDNA variants derived from primary cHL cases and cHL cell lines. (B) Endpoint PCR results of the amplified PTPN1 cDNA from laser-microdissected HRS cells of selected primary cHL cases. The PCR signal related to full-length PTPN1 mRNA (I) and the band corresponding to the PTPN1Δ6 mRNA (II) are indicated. (C) Percentage of the PTPN1Δ6 mRNA based on the whole PTPN1 mRNA levels in 24 cases from the cHL cohort measured by qPCR analyses. Three different groups are defined: group I, very low relative PTPN1Δ6 mRNA expression (cases 15, 19, 21, 25, 72, 186; red bars); group II, low relative PTPN1Δ6 mRNA expression (cases 4, 5, 6, 10, 17, 81, 82, 83, 158; blue bars); and group III, high relative PTPN1Δ6 mRNA expression (cases 9, 29, 34, 39, 52, 54, 59, 69, 86; light green bars). (D) Percentage of the PTPN1Δ6 mRNA based on the whole PTPN1 mRNA levels in the different cHL cell lines without and after stimulation with serum for 6 hours, measured by qPCR analyses. Significances are calculated in comparison with the control (0 hours) values. *P ≤ .05; **P ≤ .01; ***P ≤ .001. (E) Percentage of the PTPN1Δ6 mRNA based on the whole PTPN1 mRNA levels in other lymphoma cell lines (PMBL, ABC-DLBCL, GC-DLBCL) measured by qPCR analyses. (F) Percentage of the PTPN1Δ6 mRNA based on the whole PTPN1 mRNA levels in 8 control LCL cell lines (left) and microdissected B cells from lymph node sections. GC, germinal center; MZ, mantle zone (right part). (D-F) The dashed line marks the relative PTPN1Δ6 mRNA expression level of 0.4% observed in the group of laser-captured HRS cells with very low relative PTPN1Δ6 mRNA expression.
PTP1BΔ6 is a positive regulator of JAK/STAT signaling
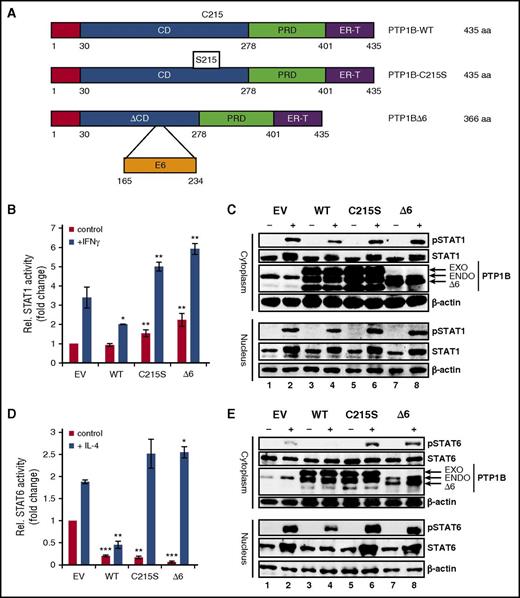
To investigate the functional relevance of the PTP1BΔ6 variant, we performed luciferase reporter assays using reporter constructs specific for either STAT1 (Figure 3B) or STAT6 (Figure 3D). HEK293 or HEK293-STAT6 cells were cotransfected with expression vectors encoding WT PTP1B, an enzymatic inactive PTP1BC215S mutant (C215S), or the PTP1BΔ6 variant (Figure 3A). Ectopic expression of PTP1BWT significantly decreased both the IFNγ-induced STAT1 activity (Figure 3B) and the IL-4-induced STAT6 activity (Figure 3D), as expected. In contrast, the PTP1BC215S mutant enhanced the IFNγ-induced STAT1 and IL-4-induced STAT6 activity. Similarly, PTP1BΔ6 also potentiated the IFNγ- and IL-4-induced STAT1 and STAT6 activity, respectively. This effect of the PTP1BΔ6 variant was also visible in western blot monitoring the phosphorylation status of STAT1 in IFNγ-stimulated HEK293 or STAT6 in IL-4-stimulated HEK293-STAT6 cells (Figure 3C,E). Although ectopic PTP1BWT caused a diminished phosphorylation of STAT1 (pSTAT1) and STAT6 (pSTAT6), we observed only a moderate increase of IFNγ-induced pSTAT1 by ectopic expression of PTP1BC215S or PTP1BΔ6 (Figure 3B). However, pSTAT6 levels were found to be distinctively increased by overexpression of either PTP1BC215S or PTP1BΔ6 (Figure 3D, upper). Taken together, the PTP1BΔ6 variant may act as a dominant negative PTP1B version.
Effect of PTP1BΔ6 on JAK/STAT signaling. (A) Schematic representation of the different PTP1B variants used in this study. CD, catalytical domain; PRD, proline-rich domain; ER-T, endoplasmic reticulum targeting domain. (B) Luciferase assay for the readout of STAT1 activity with and without (control) stimulation with IFNγ. HEK293 cells where transfected with empty vector pFLAG (EV) as a control, PTP1B WT, PTP1BC215S (C215S), and PTP1BΔ6 (Δ6) encoding vectors. Mean values and standard error of mean (SEM) are depicted. Significances are calculated in comparison with the empty vector values. *P ≤ .05; **P ≤ .01; ***P ≤ .001. (C) Cytoplasmic and nuclear extracts from HEK293 cells transfected with EV, PTP1BWT, PTP1BC215S, or PTP1BΔ6 either left unstimulated (−) or stimulated (+) with IFNγ were subjected to immunoblot analyses, using antibodies specific for pSTAT1, STAT1, PTP1B, and β-actin as a loading control. (D) Luciferase assay for STAT6 activity with and without (control) stimulation with IL-4. HEK293-ST6 cells were transiently transfected either with pFLAG-CMV2 (EV) or with PTP1BWT, PTP1BC215S, or PTP1BΔ6 vectors. Mean values and SEM are depicted. Significances are calculated in comparison with the empty vector values. *P ≤ .05; **P ≤ .01; ***P ≤ .001. (E) Cytoplasmic and nuclear extracts from HEK293-ST6 cells transfected with EV, PTP1BWT, PTP1BC215S, or PTP1BΔ6 either left unstimulated (−) or stimulated (+) with IL-4 were subjected to western blot analyses, using antibodies specific for pSTAT1, STAT1, PTP1B, and β-actin as a loading control.
Effect of PTP1BΔ6 on JAK/STAT signaling. (A) Schematic representation of the different PTP1B variants used in this study. CD, catalytical domain; PRD, proline-rich domain; ER-T, endoplasmic reticulum targeting domain. (B) Luciferase assay for the readout of STAT1 activity with and without (control) stimulation with IFNγ. HEK293 cells where transfected with empty vector pFLAG (EV) as a control, PTP1B WT, PTP1BC215S (C215S), and PTP1BΔ6 (Δ6) encoding vectors. Mean values and standard error of mean (SEM) are depicted. Significances are calculated in comparison with the empty vector values. *P ≤ .05; **P ≤ .01; ***P ≤ .001. (C) Cytoplasmic and nuclear extracts from HEK293 cells transfected with EV, PTP1BWT, PTP1BC215S, or PTP1BΔ6 either left unstimulated (−) or stimulated (+) with IFNγ were subjected to immunoblot analyses, using antibodies specific for pSTAT1, STAT1, PTP1B, and β-actin as a loading control. (D) Luciferase assay for STAT6 activity with and without (control) stimulation with IL-4. HEK293-ST6 cells were transiently transfected either with pFLAG-CMV2 (EV) or with PTP1BWT, PTP1BC215S, or PTP1BΔ6 vectors. Mean values and SEM are depicted. Significances are calculated in comparison with the empty vector values. *P ≤ .05; **P ≤ .01; ***P ≤ .001. (E) Cytoplasmic and nuclear extracts from HEK293-ST6 cells transfected with EV, PTP1BWT, PTP1BC215S, or PTP1BΔ6 either left unstimulated (−) or stimulated (+) with IL-4 were subjected to western blot analyses, using antibodies specific for pSTAT1, STAT1, PTP1B, and β-actin as a loading control.
STAT activity and proliferation is augmented by PTP1BΔ6 expression in cHL cell lines
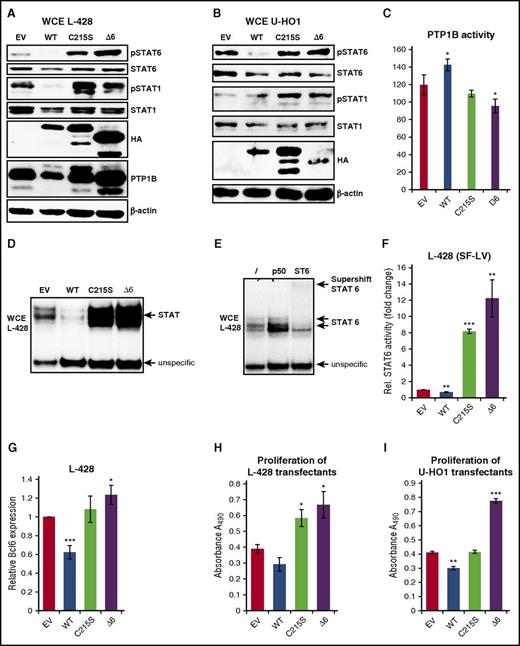
Given the positive effect of PTP1BΔ6 on JAK/STAT signaling in HEK293 cells and the essential role of the JAK/STAT pathway in cHL, we next aimed at analyzing the effect of the PTP1BΔ6 variant in cHL. For this, we generated a panel of stably transfected L-428 (Figure 4A) and U-HO1 (Figure 4B) cell clones expressing PTP1BWT, PTP1BC215S, or PTP1BΔ6. Phosphorylation of STAT6 was diminished in PTP1BWT overexpressing L-428 and U-HO1 cells, whereas PTP1BC215S or PTP1BΔ6 overexpression augmented pSTAT6 and pSTAT1 (Figure 4A-B). These changes in the phosphorylation status of STAT6 and STAT1 corresponded to increased PTP1B activity in PTP1B overexpressing L-428 cells and diminished PTP1B activity in L-428 PTP1BC215S or L-428 PTP1BΔ6 cells (Figure 4C). Furthermore, we found a decrease in STAT binding in PTP1BWT L-428 cells, whereas PTP1BC215S and PTP1BΔ6 cells displayed the opposite effect (Figure 4D). The majority of this STAT DNA binding activity was caused by STAT6 (Figure 4E), as determined by a supershift analysis. This positive effect of PTP1BΔ6 on STAT6 activity was also seen in STAT6 target gene expression analyses. Here, ectopic PTP1BWT diminished the basal STAT6 activity in L-428 cells (Figure 4F), whereas it was dramatically increased by either PTP1BC215S or PTP1BΔ6. Consistently, the expression of the STAT target gene BCL6 was found to be attenuated in PTP1BWT L-428 cells, but moderately augmented in either PTP1BC215S or PTP1BΔ6 L-428 cells (Figure 4G).
Stable expression of PTP1BΔ6 modulates JAK/STAT signaling and proliferation. (A-B) Immunoblot analysis of WCE from (A) L-428 cells or (B) U-HO1 stably expressing PTP1BWT, PTP1BC215S, and PTP1BΔ6 or control cells (EV) using the indicated antibodies. (C) PTP1B phosphatase activity in the different L-428 cell clones determined by a PTP1B phosphatase assay. Significances are calculated in comparison with the empty vector values. *P ≤ .05; **P ≤ .01; ***P ≤ .001. (D) Electrophoretic mobility shift assay to determine STAT DNA binding activity with WCE from L428 cells expressing ectopic PTP1BWT, PTP1BC215S, PTP1BΔ6, or L-428 control cells (EV). (E) Supershift analysis of WCE from untreated L-428 control cells using no antibody (lane 1), a NF-κB p50-specific antibody (lane 2, negative control), or an anti-STAT6 specific antibody (lane 3). (F) STAT6 luciferase reporter activity in L-428 cells stably expressing PTP1BWT, PTP1BC215S, and PTP1BΔ6 and in L-428 control cells (EV). Mean values and SEM are depicted. Significances are calculated in comparison with the empty vector values. *P ≤ .05; **P ≤ .01; ***P ≤ .001. (G) BCL6 mRNA expression levels in the different L-428 cell clones determined by qRT-PCR analysis. Mean values and SEM are depicted. Significances are calculated in comparison with the empty vector values. *P ≤ .05; **P ≤ .01; ***P ≤ .001. (H-I) Proliferation of the different stable L-428 (H) or U-HO1 (I) cell clones determined by MTS assay. Mean values and SEM are depicted. Significances are calculated in comparison with the empty vector values. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
Stable expression of PTP1BΔ6 modulates JAK/STAT signaling and proliferation. (A-B) Immunoblot analysis of WCE from (A) L-428 cells or (B) U-HO1 stably expressing PTP1BWT, PTP1BC215S, and PTP1BΔ6 or control cells (EV) using the indicated antibodies. (C) PTP1B phosphatase activity in the different L-428 cell clones determined by a PTP1B phosphatase assay. Significances are calculated in comparison with the empty vector values. *P ≤ .05; **P ≤ .01; ***P ≤ .001. (D) Electrophoretic mobility shift assay to determine STAT DNA binding activity with WCE from L428 cells expressing ectopic PTP1BWT, PTP1BC215S, PTP1BΔ6, or L-428 control cells (EV). (E) Supershift analysis of WCE from untreated L-428 control cells using no antibody (lane 1), a NF-κB p50-specific antibody (lane 2, negative control), or an anti-STAT6 specific antibody (lane 3). (F) STAT6 luciferase reporter activity in L-428 cells stably expressing PTP1BWT, PTP1BC215S, and PTP1BΔ6 and in L-428 control cells (EV). Mean values and SEM are depicted. Significances are calculated in comparison with the empty vector values. *P ≤ .05; **P ≤ .01; ***P ≤ .001. (G) BCL6 mRNA expression levels in the different L-428 cell clones determined by qRT-PCR analysis. Mean values and SEM are depicted. Significances are calculated in comparison with the empty vector values. *P ≤ .05; **P ≤ .01; ***P ≤ .001. (H-I) Proliferation of the different stable L-428 (H) or U-HO1 (I) cell clones determined by MTS assay. Mean values and SEM are depicted. Significances are calculated in comparison with the empty vector values. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
Cell growth was diminished in L-428 and U-HO1 cells ectopically expressing PTP1BWT (Figure 4H-I), whereas expression of PTP1BΔ6 in L-428 cells, and even more in U-HO1 cells, caused a marked increase in cell proliferation. However, the results for PTP1BC215S appeared to be nonuniform. Although L-428 displayed a moderately increased proliferation, growth of U-HO1 PTP1BC215S cells remained unchanged.
Collectively, PTP1BΔ6 acts as a dominant negative PTP1B variant in cHL, having a positive effect on JAK/STAT signaling and cell proliferation.
PTP1BΔ6 protects L-428 cells against cytotoxic agents
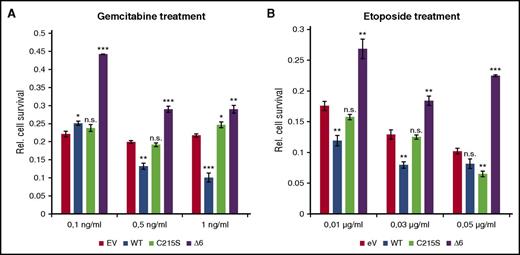
Because constitutive STAT activity is a critical survival mechanism in cHL, the effect of the different PTP1B variants on the cytotoxicity of 2 chemotherapeutical agents, gemcitabine and etoposide, was analyzed. The reported IC50 value of gemcitabine and etoposide for L-428 cells proved very low, with less than 0.01 µg/mL for gemcitabine and 0.13 µg/mL for etoposide.19 This is in good agreement with their strong lethal effect on L-428 cells, leading to an approximately 80% reduced viability already with the lowest concentrations of either gemcitabine (Figure 5A) or etoposide (Figure 5B). Overexpression of PTP1BWT further decreased L-428 cell numbers after treatment with 0.5 or 1 ng/mL gemcitabine or 0.01 and 0.03 µg/mL etoposide. In contrast, ectopically expressed PTP1BΔ6 partially protected L-428 cells after 0.1 ng/mL gemcitabine or 0.01 µg/mL etoposide treatment, as displayed by reduced cytotoxicity (45% in case of gemcitabine and 28% in case of etoposide) of these agents (Figure 5). Remarkably, overexpression of PTP1BC215S affected the viability of L-428 cells only moderately if at all, highlighting the unique protective effect of the PTP1BΔ6 variant in L-428 cells.
PTP1BΔ6 protects L-428 cells against cytotoxic agents. (A) Proliferation of the different stable L-428 cell clones treated with the indicated concentrations of gemcitabine for 24 hours, determined by MTS assay. (B) Proliferation of the different stable L-428 cell clones treated with the indicated concentrations of etoposide for 24 hours determined by MTS assay. Proliferation of solvent-treated L-428 cell clones was set to 1 arbitrarily. Depicted is the relative proliferation of the gemcitabine-treated cells. All treatments were performed in triplicates. Mean values and SEM are depicted. Significances are calculated in comparison with the empty vector values. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
PTP1BΔ6 protects L-428 cells against cytotoxic agents. (A) Proliferation of the different stable L-428 cell clones treated with the indicated concentrations of gemcitabine for 24 hours, determined by MTS assay. (B) Proliferation of the different stable L-428 cell clones treated with the indicated concentrations of etoposide for 24 hours determined by MTS assay. Proliferation of solvent-treated L-428 cell clones was set to 1 arbitrarily. Depicted is the relative proliferation of the gemcitabine-treated cells. All treatments were performed in triplicates. Mean values and SEM are depicted. Significances are calculated in comparison with the empty vector values. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
PTP1BΔ6 impedes the normal PTP1B function by dimer formation
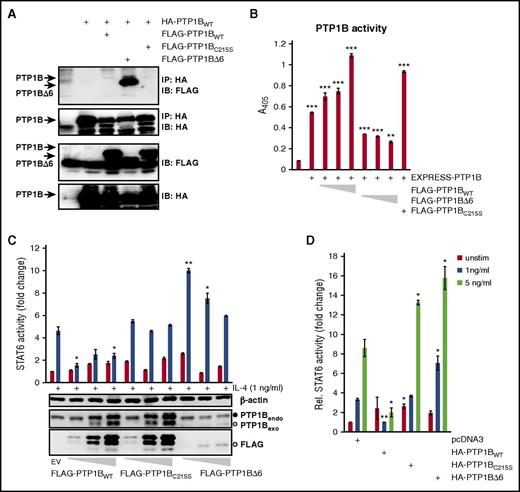
To get an insight into the mechanistic basis of the positive PTP1BΔ6 effects, we performed a coimmunoprecipitation analysis to look for a possible interaction of FLAG-tagged PTP1BWT, PTP1BC215S, and PTP1BΔ6 with a HA-tagged PTP1BWT.20,21 For this, HEK293 cell were transiently transfected with an expression vector encoding HA-tagged PTP1BWT either alone or in combination with expression vectors for FLAG-tagged PTP1BWT, PTP1BC215S, or PTP1BΔ6 (Figure 6A). The HA-PTP1BWT containing protein complexes were isolated by anti-HA immunoprecipitation, and the interaction with the FLAG-PTP1B variants was determined by an anti-FLAG immunoblot analysis. Only small amounts of FLAG-PTP1BWT or FLAG-PTP1BC215S were coimmunoprecipitated by HA-PTP1BWT (Figure 6A, lanes 3 and 5). In contrast, FLAG-PTP1BΔ6 was copurified by the anti-HA immunoprecipitation very efficiently (Figure 6A, lane 4). Consistently, addition of PTP1BΔ6 hampered the phosphatase activity of PTP1BWT in an in vitro assay (Figure 6B).
PTP1BΔ6:PTP1BWT dimer formation attenuates PTP1B activity. (A) Protein complex formation of HA-PTP1BWT with FLAG-PTP1BWT, FLAG-PTP1BΔ6, or FLAG-PTP1BC215S. WCE from HEK293 cells ectopically expressing HA-PTP1BWT either alone or in combination with FLAG-PTP1BWT, FLAG-PTP1BΔ6, or FLAG-PTP1BC215S were subjected to an immunoprecipitation analysis using an anti-HA antibody. Coprecipitated FLAG-PTP1B variants were visualized by an anti-FLAG immunoblot analysis. (B) Phosphatase assay with EXPRESS-tagged PTP1BWT alone or together with HA-PTP1BC215S, or increasing amounts of HA-PTP1BWT, or HA-PTP1BΔ6 ectopically expressed in HEK293 cells, as indicated. pEXPRESS (EV) serves as control. Mean values and SEM are depicted. Significances are calculated in comparison with the empty vector values. *P ≤ .05; **P ≤ .01; ***P ≤ .001. (C) Luciferase assay to determine the STAT6 activity in HEK293-ST6 cells with and without (control) stimulation with IL-4. The cells were transiently transfected with 10, 50, or 100 ng FLAG-PTP1BWT, FLAG-PTP1BC215S, or FLAG-PTP1BΔ6. The resulting WCE were additionally tested for the expression of exogenous PTP1B (anti-FLAG IB) or overall PTP1B (anti-PTP1B IB). The full black circle indicates the position of the endogenous PTP1B, the open circle indicates the position of the exogenous PTP1BΔ6 (lower-middle, last 3 lanes). The membrane was reprobed, using an anti-β-actin antibody to ensure equal protein loading. Mean values and SEM are depicted. Significances are calculated in comparison with the empty vector values. *P ≤ .05; **P ≤ .01; ***P ≤ .001. (D) Luciferase assay for STAT6 activity with either unstimulated cells (control) or cells stimulated with either 1 ng/ml or 5 ng/ml IL-4. HEK293-ST6 cells were transiently transfected with 100 ng HA-PTP1BWT, HA-PTP1BΔ6, or HA-PTP1BC215S. pcDNA3.1 (EV) serves as control. Mean values and SEM are depicted. Significances are calculated in comparison with the empty vector values. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
PTP1BΔ6:PTP1BWT dimer formation attenuates PTP1B activity. (A) Protein complex formation of HA-PTP1BWT with FLAG-PTP1BWT, FLAG-PTP1BΔ6, or FLAG-PTP1BC215S. WCE from HEK293 cells ectopically expressing HA-PTP1BWT either alone or in combination with FLAG-PTP1BWT, FLAG-PTP1BΔ6, or FLAG-PTP1BC215S were subjected to an immunoprecipitation analysis using an anti-HA antibody. Coprecipitated FLAG-PTP1B variants were visualized by an anti-FLAG immunoblot analysis. (B) Phosphatase assay with EXPRESS-tagged PTP1BWT alone or together with HA-PTP1BC215S, or increasing amounts of HA-PTP1BWT, or HA-PTP1BΔ6 ectopically expressed in HEK293 cells, as indicated. pEXPRESS (EV) serves as control. Mean values and SEM are depicted. Significances are calculated in comparison with the empty vector values. *P ≤ .05; **P ≤ .01; ***P ≤ .001. (C) Luciferase assay to determine the STAT6 activity in HEK293-ST6 cells with and without (control) stimulation with IL-4. The cells were transiently transfected with 10, 50, or 100 ng FLAG-PTP1BWT, FLAG-PTP1BC215S, or FLAG-PTP1BΔ6. The resulting WCE were additionally tested for the expression of exogenous PTP1B (anti-FLAG IB) or overall PTP1B (anti-PTP1B IB). The full black circle indicates the position of the endogenous PTP1B, the open circle indicates the position of the exogenous PTP1BΔ6 (lower-middle, last 3 lanes). The membrane was reprobed, using an anti-β-actin antibody to ensure equal protein loading. Mean values and SEM are depicted. Significances are calculated in comparison with the empty vector values. *P ≤ .05; **P ≤ .01; ***P ≤ .001. (D) Luciferase assay for STAT6 activity with either unstimulated cells (control) or cells stimulated with either 1 ng/ml or 5 ng/ml IL-4. HEK293-ST6 cells were transiently transfected with 100 ng HA-PTP1BWT, HA-PTP1BΔ6, or HA-PTP1BC215S. pcDNA3.1 (EV) serves as control. Mean values and SEM are depicted. Significances are calculated in comparison with the empty vector values. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
To explore whether even low PTP1BΔ6 levels would affect JAK-STAT signaling, we performed luciferase reporter analyses with increasing amounts of PTP1BWT, PTP1BC215S, and PTP1BΔ6 (Figure 6C). Again, we observed a distinctively increased IL-4-induced STAT6 activity, even with the lowest amount of PTP1BΔ6, which was hardly detectable in an anti-PTP1B western blot analysis. Similarly, only the doxycycline-induced low expression of PTP1BΔ6, but not of PTP1BC215S, augmented the STAT activity in a KM-H2 cell model (supplemental Figure 4). As these data suggest that PTP1BΔ6 confers an increased sensitivity to the JAK-STAT signaling, we next measured the effect of PTP1BΔ6 on suboptimal IL-4-triggering of JAK-STAT activity (Figure 6D). Indeed, PTP1BΔ6 coexpression boosted the JAK-STAT activity even at low concentrations of IL-4.
Discussion
Constitutively activated JAK/STAT signaling is crucial for the propagation and survival of HRS cells, and recurrent mutations in SOCS1 and PTPN1 participate in this process. Here, we identified a set of novel PTPN1 mRNA variants in primary cHL tumor samples. Although the PTPN1Δ2-4 and the PTPN1Δ2-8 variants isolated from the HRS cells of samples 5 and 9 or 3 correspond to those observed in the cHL cell lines SUP-HD1 and U-HO1, respectively, which come up as a consequence of mutations of PTPN1,5 another PTPN1 mRNA variant identified in cHL sample 1 with remaining intron 9 to 10 sequences was identified previously as an alternatively spliced PTPN1 mRNA variant observed in epidermal growth factor or serum stimulated human fibroblasts.12 Similarly, the PTPN1Δ6 mRNA variant is a novel splice variant and not a product of a mutated PTPN1, as it was detectable in various cHL cell lines, in laser-captured HRS cells, and in normal B cells (Figure 2C-D,F), and the relative ratio of PTPN1Δ6 mRNA expression levels were increased on serum stimulation in these cHL cell lines (Figure 2C).
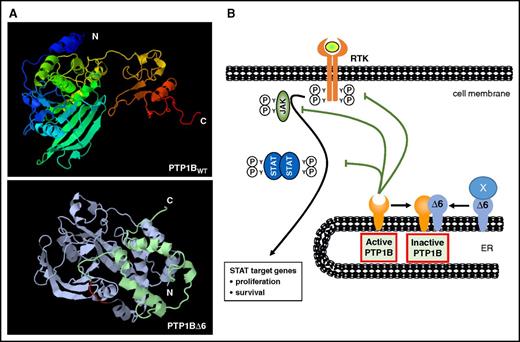
The formation of PTP1B variants appears to be a widely used mechanism to alter the functions of PTP1B. In addition to signal-induced alternative splicing, a calpain-mediated processing of the preexisting full-length PTP1B protein by limited proteolysis has been shown to affect cellular PTP1B functions, subcellular localization, and substrate profiles of this phosphatase.22 The lack of exon 6 in the PTPN1 mRNA causes the loss of aa 165 to 234 (Figure 2A) leading to a PTP1B variant with distinct structural and functional alterations (Figure 7). The most prominent functional change is its enzymatic inactivity as a wide-spanning part of the phosphatase domain including the critical Cys215 residue is deleted. This might explain the increased IFNγ- or IL-4-induced STAT activity in HEK293 or HEK293-STAT6 cells (Figure 3) and the augmented basal STAT activity in the cHL cell lines L-428 and U-HO1 (Figure 4), which are similar to the effects of the enzymatic inactive PTP1BC215S mutant. However, the loss of aa 165 to 234 not only eliminates the enzymatic domain but also causes a protein with a distinctively different structure (Figure 7). This might explain why PTP1BΔ6 harbors unique features not observed with PTP1BC215S. Thus, cytotoxic effects of etoposide or gemcitabine on L-428 cells remained unaltered by PTP1BC215S expression, whereas PTP1BΔ6 partially protected these cells against their toxicity (Figure 5). The basis for these differences might be the increased capacity of the PTP1BΔ6 variant to form dimers with PTP1BWT in comparison with PTP1BWT homodimer formation or PTP1BWT:PTP1BC215S heterodimerization (Figure 6). A dimerization of PTP1B has been reported previously.20,21 However, neither the domains required for the homodimerization have been defined, nor has the functional relevance of PTP1B homodimerization been clarified. Yet, it was shown that dimerization and active site blockage are physiologically important mechanisms for down-regulating the catalytic activity of the receptor-like protein-tyrosine phosphatase-α.23 Similarly, the increased PTP1BΔ6:PTP1BWT interaction attenuates the PTP1B activity, as shown by an in vitro phosphatase assay (Figure 6B). However, interaction with and downregulation of PTP1BWT activity seems not to be the only molecular mechanism by which PTP1BΔ6 exerts its oncogenic effects, as an increased STAT1 phosphorylation and an augmented proliferation were also seen on ectopic PTP1BΔ6 expression in the PTP1B-deficient cHL cell line U-HO1 (Figure 4B,I). For instance, interaction with p130CAS, a regulator of the RAS-MAPK signaling, was shown to be mediated by a carboxy-terminal proline-rich region, which is still present in PTP1BΔ6.24 Moreover, the interaction and functional modulation of additional novel partners by PTP1BΔ6 is also conceivable. The suggested mode of action of the PTP1BΔ6 variant is summarized in a schematic model (Figure 7B).
Proposed model of PTP1BΔ6 action in cHL. (A) Structural models of PTP1BWT (top) and PTP1BΔ6 (bottom) generated by the SEQALSV (Sequence Alignment-based Sequence Variability) and AS2TS (amino acid sequence into tertiary structure) online software tools. (B) Schematic representation of the potential mode of action of FLAG-PTP1BΔ6. FLAG-PTP1BΔ6 binds and diminishes the negative effects of PTP1BWT. In addition, PTP1BΔ6 forms complexes with yet unknown interaction partners (labeled as X). ER, endoplasmic reticulum; RTK, receptor tyrosine kinase.
Proposed model of PTP1BΔ6 action in cHL. (A) Structural models of PTP1BWT (top) and PTP1BΔ6 (bottom) generated by the SEQALSV (Sequence Alignment-based Sequence Variability) and AS2TS (amino acid sequence into tertiary structure) online software tools. (B) Schematic representation of the potential mode of action of FLAG-PTP1BΔ6. FLAG-PTP1BΔ6 binds and diminishes the negative effects of PTP1BWT. In addition, PTP1BΔ6 forms complexes with yet unknown interaction partners (labeled as X). ER, endoplasmic reticulum; RTK, receptor tyrosine kinase.
Increased affinity to conventional PTP1B interaction partners or binding to alternative interaction partners might be the reason for the functional effect of the PTP1BΔ6 variant, despite its low expression levels in comparison with the WT PTP1B. A similar situation was observed for alternatively spliced variants of the androgen receptor in prostate cancer. ARV mRNA levels range from 0.1% to 2.5% of AR-FL mRNA levels, yet ARVs display high transcriptional activities.25
Accumulating recently published studies highlight the general importance of tumor-specific expression of novel or otherwise underrepresented splice variants. This has been reported for mRNAs for TP53, BARD1, and AR (reviewed in Chen and Weiss26 ). Some of the corresponding protein variants obtain gains of function, even turning a tumor suppressor such as p53 into a protein with oncogenic properties. Moreover, an increased formation of aberrantly spliced mRNA variants is a known feature of cHL,27 although the existence of splice variants of PTP1B in cHL, either as consequence of PTPN1 mutations or by disregulated splicing machinery, has not been reported previously.
Taken together, PTP1BΔ6 is a novel PTP1B splice variant augmenting JAK/STAT activation and proliferation and survival of cHL cells. Although very low levels of PTP1BΔ6, as detectable in normal B cells, might function as a kind of rheostat, fine-tuning the PTP1B activity, the consistently higher levels as observed in HRS cells are a novel oncogenic functional element in the oncobiology of cHL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Michaela Buck for technical assistance and Alexej Ushmorov (Institute for Physiological Chemistry, Ulm University) for his help with the lentiviral transduction. The authors are grateful for the help with the mRNA extraction of microdissected HRS cells of Karl Hofmann and Ulrike Kostezka.
This work was supported by funds from the Deutsche Forschungsgemeinschaft (MO 384/6-1 [P.M.] and MA2367/6-1 [R.M.]).
Authorship
Contribution: R.M. and P.M. designed the study and wrote the paper; R.M., T.F.E.B., and P.M. designed and analyzed the experiments; M.Z. performed and analyzed the experiments and wrote the paper; and R.M., A.M., S.B., I.M., J.H., B.R., S.W., and K.D. performed and analyzed the experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter Möller, Institute of Pathology, Ulm University, Albert-Einstein-Allee 23, D-89070 Ulm, Germany; e-mail: peter.moeller@uniklinik-ulm.de.
References
Author notes
M.Z. and R.M. contributed equally to this study.