Key Points
Platelets are not killer cells of blood-stage Plasmodium parasites.
Platelets are not required to activate the protective immune response to blood-stage Plasmodium infection in mice.
Abstract
Clinical studies indicate that thrombocytopenia correlates with the development of severe falciparum malaria, suggesting that platelets either contribute to control of parasite replication, possibly as innate parasite killer cells or function in eliciting pathogenesis. Removal of platelets by anti-CD41 mAb treatment, platelet inhibition by aspirin, and adoptive transfer of wild-type (WT) platelets to CD40-KO mice, which do not control parasite replication, resulted in similar parasitemia compared with control mice. Human platelets at a physiologic ratio of 1 platelet to 9 red blood cells (RBCs) did not inhibit the in vitro development or replication of blood-stage Plasmodium falciparum. The percentage of Plasmodium-infected (iRBCs) with bound platelets during the ascending parasitemia in Plasmodium chabaudi– and Plasmodium berghei–infected mice and the 48-hour in vitro cycle of P falciparum was <10%. P chabaudi and P berghei iRBCs with apoptotic parasites (TdT+) exhibited minimal platelet binding (<5%), which was similar to nonapoptotic iRBCs. These findings collectively indicate platelets do not kill bloodstage Plasmodium at physiologically relevant effector-to-target ratios. P chabaudi primary and secondary parasitemia was similar in mice depleted of platelets by mAb-injection just before infection, indicating that activation of the protective immune response does not require platelets. In contrast to the lack of an effect on parasite replication, adoptive transfer of WT platelets to CD40-KO mice, which are resistant to experimental cerebral malaria, partially restored experimental cerebral malaria mortality and symptoms in CD40-KO recipients, indicating platelets elicit pathogenesis and platelet CD40 is a key molecule.
Introduction
A hallmark of blood-stage Plasmodium infection in humans is the development of thrombocytopenia and a procoagulant state that is most pronounced in Plasmodium falciparum (Pf) infections, the most virulent of the 5 species of Plasmodium infecting humans.1,2 Levels of thrombocytopenia and procoagulant state are elevated in severe Pf malaria compared with uncomplicated malaria patients and uninfected controls,1,2 suggesting that thrombocytopenia contributes to disease. However, there is still considerable uncertainty about platelets’ role in malaria. Thrombocytopenia could lead to increased parasite replication by: (1) decreased killing of parasites or (2) decreased activation of the protective immune response controlling parasite replication.
In support of increased parasite replication contributing to severe malaria, 2 groups reported that platelets function as a critical component of innate immunity controlling parasite replication by binding to and directly killing parasites within red blood cells (RBCs).3-5 Based on increased mortality of aspirin-treated, Plasmodium chabaudi (Pc)–infected mice and inhibition of in vitro killing of Pf-infected RBCs (iRBCs) by aspirin,4 Greenbaum6 proposed to change clinical practice by contraindicating widely used nonsteroidal anti-inflammatory agents with antiplatelet activity, such as aspirin, in malaria patients.
In contrast to reported iRBC killing by platelets,3-5 we reported that platelet depletion by antibody injection does not affect Plasmodium berghei ANKA (PbA) parasitemia,7,8 which elicits experimental cerebral malaria (eCM) in mice. Early but not late platelet depletion protects against eCM pathogenesis by ameliorating the inflammatory response.7,8 Here, we determine whether platelets are innate killers of blood-stage Plasmodium and/or regulate either the protective or pathogenic immune response during malaria and report that there is no evidence to support physiologic platelet killing of blood-stage Plasmodium. Moreover, platelets do not lead to a protective immune response that clears blood-stage Plasmodium infection, but do activate a pathogenic response to infection.
Methods
Ethics statement
The Institutional Animal Care and Use Committee of La Jolla Infectious Disease Institute approved all protocols and procedures.
Mouse studies
There are differences between humans and mice and between Plasmodium species infecting humans and those infecting mice, indicating caution is needed in extrapolating results to humans.9 Nevertheless, key hallmarks of malarial thrombocytopenia, immunity, and cerebral malaria (CM) pathogenesis appear to be conserved.10 We used 3 different strains of Plasmodium that infect mice using standard protocols.11,12 The iRBC inoculum details (0.2 mL in phosphate-buffered saline [PBS] injected IV) and logic behind the selection of the 3 strains are summarized in supplemental Table 1. Day 0PI are uninfected control mice. Between 200 and 1000 RBCs were counted in Giemsa-stained thin blood films in experimental animals to assess parasitemia.
Treatments.
Aspirin (acetylsalicylic acid; Sigma, St. Louis, MO) was injected intraperitoneally (IP) in 0.2 mL saline (25 mg/kg). Platelets were depleted by IP injection with 0.1 mg platelet-depleting anti-CD41 mAb in 0.1 mL PBS (MWReg40); control mice were injected with rat IgG mAb (Affymetrix, San Diego, CA). Immunodeficient CD40-KO mice and C57BL/6 wild-type (WT) controls were cured of Plasmodium chabaudi adami (Pca) infection with trimethoprim (0.4 mg/mL) and sulfamethoxazole (1.2 mg/mL) ad libitum for 1 week in drinking water after WT controls had suppressed their parasitemia to undetectable levels (from >day 28PI). Cure was confirmed by 3 negative thin blood films.
Platelet analysis and isolation.
Platelets and RBCs were counted by flow cytometry in 1 µL of tail vein blood as described previously8 ; the number of platelets/mL blood, platelet activation, and response was similar in tail vein blood compared with blood obtained by intracardiac puncture or via retro-orbital plexus.8
Purified platelets for adoptive transfer were obtained by centrifugation of whole blood at 50×g for 20 minutes to generate platelet-rich plasma (PRP). PRP was centrifuged at 800×g for 10 minutes and platelets resuspended in Tyrode’s buffer at 0.2 × 109 in 0.2 mL saline for injection.
Flow cytometry
All antibody incubations were performed at room temperature using antibodies purchased from Affymetrix. Anti-CD41-phycoerythrin (PE) fluorescence–labeled platelets, and anti-Ter119-PE-cyanin7 (PE-Cy7)–labeled erythrocytes. Cells were resuspended in 0.5 mL PBS with 2.5 × 104 counting beads (Spherotech, Lake Forest, IL), and fluorescence intensities of the cells were acquired on an Accuri (Becton Dickinson, San Diego, CA) as described.12
The Pca high-virulence (HV) and human platelet interaction with P falciparum iRBCs were performed on an ImageStream MkII flow cytometer (EMD Millipore, Seattle, WA), which distinguishes coincident events from close proximity/adhesion.
Fluorescence labeling of thin blood films
Thin blood films made on glass slides were fixed for 1 minutes in methanol (Sigma). Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay was performed following the manufacturer’s instructions (EMD Millipore, Temecula, CA). Platelets were labeled with anti-CD41-APC mAb. Parasite DNA and RNA were fluorescence labeled with 12 µM ethidium bromide (Fisher Scientific, Fair Lawn, NJ) in PBS for 15 minutes. Slides were mounted with Vectashield Mounting Media (Vector Laboratories, Burlingame, CA).
Pf culture and analysis
Blood-stage Pf strains DD2, FCR3, and NK54 were cultured in vitro as described.15,16 Blood was obtained from 3 volunteers who had not taken anti-inflammatory medications for >2 weeks before venipuncture. The blood was collected into 5 mL citrate vacutainers using a 21-G needle (Becton Dickinson), processed into PRP, washed in Tyrode’s buffer, resuspended in complete media, and added at a ratio of 1 platelet per 9 RBCs to the Pf cultures. Parasitemia of cultures with and without platelets and the percentage of RBCs with an adherent platelet were assessed at 0, 2, 4, 6, 24, and 48 hours using imaging flow cytometry. The thrombin (Fisher Scientific, Chronolog) dose response (CD62P levels) was assessed for each donor at the above selected time points.
Statistical analysis
Analysis of variance with the Prism program (GraphPad) with Tukey’s post-hoc test was performed to statistically compare all measurements with a P value cutoff of .05. The mean and standard error of the mean of the results are reported in text and figures. Survival curves are compared with nonparametric log-rank test with a P cutoff of .05 with the “neurologic period” for the development of eCM occurring on days 6 to 12 in C57BL/6 mice.
Results
Platelet killing of Plasmodium
Malaria in both humans and mice results in profound thrombocytopenia, occurring during the period of ascending patent parasitemia from day 4 to 7PI for Pca in mice. Non-lethal Pca, low virulence (LV), and HV elicit exponential ascending parasitemia until close to peak parasitemia, with slopes varying depending on parasite virulence (supplemental Table 1); after peak, parasitemia declines to undetectable levels (<0.1%) as a result of the activation of adaptive immune responses.11,17,18 When plotted linearly, thrombocytopenia coincides with patent parasitemia and its exponential rise, suggesting that parasite replication may be increased when circulating platelet numbers are reduced.4
Onset of thrombocytopenia exhibits no detectable effect on parasite replication.
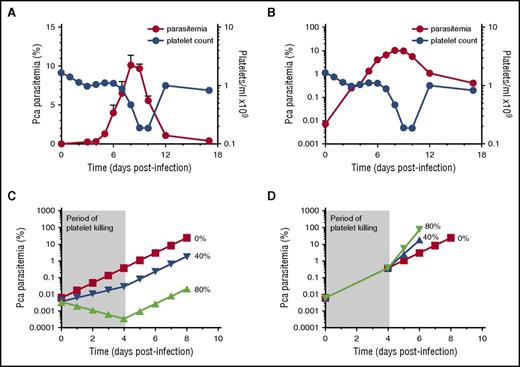
To test whether the rate of change of parasitemia is altered when thrombocytopenia occurs (and hence decreased platelet killing), we infected mice with Pca LV. The increase in parasitemia is concurrent with the onset of thrombocytopenia when plotted on a linear scale (Figure 1A).
No change in slope of log(%parasitemia) after onset of thrombocytopenia that would suggest platelets affecting parasite replication in resolving, non-eCM Pca LV infection. Parasitemia denoted by red circles (left y-axis) with corresponding platelet counts/mL in blue circles (right y-axis) for panels A-B. (A) Parasitemia of Pca-infected mice (n = 10) plotted on a linear scale with latent parasitemia coinciding with thrombocytopenia. (B) Parasitemia in panel A plotted on logarithmic scale and no change in slope occurs at the onset of thrombocytopenia on day 4 PI. This experiment was replicated 3 times. (C) Modeling of log of %(parasitemia) at selected rates of parasite killing by platelets between day 0 and 4 PI assuming sequestration does not affect parasitemia. We model the Pca LV parasitemia in a 20 g mouse, which calculates the initial parasitemia (supplemental Table 1). Platelet killing of Pf-iRBCs is ∼80%,4,5 and we compare this with a more conservative 40% killing and no killing. At initiation of LV infection, the 20 g mouse has: 1 × 106 iRBCs; 1.6 × 1010 RBCs; 1.6 × 109 platelets; and a platelet effector to iRBC target ratio >1000, resulting in ∼80% killing of iRBCs by platelets based on in vitro killing of Pf-iRBCs.4,5 At the onset of thrombocytopenia (day 4PI: ∼0.5% parasitemia; 1.6 × 1010 RBCs, and 1.6 × 109 platelets), the platelet effector-to-target ratio is 20:1; theoretically, platelet killing is minimal below 160:1.5 The period of platelet killing of iRBCs is therefore between day 0 and 4 PI (shaded, labeled “Period of platelet killing”). During ascending parasitemia (day 7PI: ∼6% parasitemia; 1.6 × 1010 RBCs; and ∼1.6 × 108 platelets), the ratio is 1:600. The parasite replicates each night producing new progeny, and the MOI during period of no killing and determined from panel B is 2.8. If each day during the platelet killing period, 80% or 40% of iRBCs are killed by platelets, then MOI declines to 0.6 (20% of 2.8) and 1.7 (60% of 2.8), respectively. The measured parasitemia (B) fits 0% line rather than 40% or 80% killing. (D) Modeling of log(%parasitemia) assuming similar degrees of iRBC sequestration throughout ascending parasitemia and platelet killing until day 4 PI as described above in panel C. Because the slope of log(%parasitemia) is linear, parasitemia likely reflects overall parasite load. The slope of log(%parasitemia) during period of platelet killing from day 0 to 4PI is 2.8 (B); this estimates effective rate of parasitemia increase with sequestration. Because platelet killing is minimal beyond day 4 PI, this rate of parasitemia increase should increase markedly with an MOI from 2.8 (0% killing) to 14 (80% killing) and 7 (40% killing) after day 4 PI. The measured parasitemia (B) clearly fits 0% platelet killing best. The modeling indicates that the slope of log(%parasitemia) should change markedly if platelet killing occurs.
No change in slope of log(%parasitemia) after onset of thrombocytopenia that would suggest platelets affecting parasite replication in resolving, non-eCM Pca LV infection. Parasitemia denoted by red circles (left y-axis) with corresponding platelet counts/mL in blue circles (right y-axis) for panels A-B. (A) Parasitemia of Pca-infected mice (n = 10) plotted on a linear scale with latent parasitemia coinciding with thrombocytopenia. (B) Parasitemia in panel A plotted on logarithmic scale and no change in slope occurs at the onset of thrombocytopenia on day 4 PI. This experiment was replicated 3 times. (C) Modeling of log of %(parasitemia) at selected rates of parasite killing by platelets between day 0 and 4 PI assuming sequestration does not affect parasitemia. We model the Pca LV parasitemia in a 20 g mouse, which calculates the initial parasitemia (supplemental Table 1). Platelet killing of Pf-iRBCs is ∼80%,4,5 and we compare this with a more conservative 40% killing and no killing. At initiation of LV infection, the 20 g mouse has: 1 × 106 iRBCs; 1.6 × 1010 RBCs; 1.6 × 109 platelets; and a platelet effector to iRBC target ratio >1000, resulting in ∼80% killing of iRBCs by platelets based on in vitro killing of Pf-iRBCs.4,5 At the onset of thrombocytopenia (day 4PI: ∼0.5% parasitemia; 1.6 × 1010 RBCs, and 1.6 × 109 platelets), the platelet effector-to-target ratio is 20:1; theoretically, platelet killing is minimal below 160:1.5 The period of platelet killing of iRBCs is therefore between day 0 and 4 PI (shaded, labeled “Period of platelet killing”). During ascending parasitemia (day 7PI: ∼6% parasitemia; 1.6 × 1010 RBCs; and ∼1.6 × 108 platelets), the ratio is 1:600. The parasite replicates each night producing new progeny, and the MOI during period of no killing and determined from panel B is 2.8. If each day during the platelet killing period, 80% or 40% of iRBCs are killed by platelets, then MOI declines to 0.6 (20% of 2.8) and 1.7 (60% of 2.8), respectively. The measured parasitemia (B) fits 0% line rather than 40% or 80% killing. (D) Modeling of log(%parasitemia) assuming similar degrees of iRBC sequestration throughout ascending parasitemia and platelet killing until day 4 PI as described above in panel C. Because the slope of log(%parasitemia) is linear, parasitemia likely reflects overall parasite load. The slope of log(%parasitemia) during period of platelet killing from day 0 to 4PI is 2.8 (B); this estimates effective rate of parasitemia increase with sequestration. Because platelet killing is minimal beyond day 4 PI, this rate of parasitemia increase should increase markedly with an MOI from 2.8 (0% killing) to 14 (80% killing) and 7 (40% killing) after day 4 PI. The measured parasitemia (B) clearly fits 0% platelet killing best. The modeling indicates that the slope of log(%parasitemia) should change markedly if platelet killing occurs.
Thrombocytopenia starts on day 4PI and becomes marked during ascending and peak parasitemia (Figure 1A). Further, in vitro cultured human platelets exhibit >80% killing of Pf-iRBC with platelet-to-iRBC target ratios >160:14,5 and <10% killing at 10:15 ; platelets should exhibit similar levels of killing in vivo. Based on these platelet-iRBC ratios, the period of platelet killing of Pca-iRBCs was between day 0 and 4PI (Figure 1); beyond day 4PI, minimal platelet killing of Pca-iRBCs occurs.
The log(parasitemia) at each day of infection from day 0 to day 7PI (ascending parasitemia) increases in a straight line with slope of 0.45 ± 0.03 (R2 = 0.99) (Figure 1B). This slope determines the multiplicity of infection (MOI) for Pca, which replicates nightly to produce new iRBCs. Each cycle (ie, day) ∼3 merozoites (100.45 = 2.8) invade a new RBC and develop into viable circulating iRBCs. During ascending parasitemia, there was no detectable change in slope in the period of platelet killing of Pca-iRBCs (day 0-4PI) and the period of no killing (>day 4PI). Hence, the MOIs were identical at 2.8.
Models indicate that thrombocytopenia should affect slope of log(parasitemia) when platelet killing diminishes.
To predict the effects of platelet killing of iRBCs on Pca parasitemia, we modeled its potential effects on parasitemia. There are 2 possible scenarios for parasitemia: (1) iRBC sequestration does not markedly affect parasitemia and (2) iRBC sequestration decreases parasitemia. We model 2 levels of platelet killing of iRBCs: 80% above platelet-iRBC threshold of 160 (day 0-4PI)4,5 or a conservative estimate of 40%.
With minimal iRBC sequestration in organs, parasitemia reflects the total number of iRBCs. During the period of platelet killing, only 20%, 60%, or 100% of iRBCs survive for 80%, 40%, and 0% killing, respectively, whereas MOIs are 0.6, 1.7, and 2.8, respectively. The parasitemia on day 0PI is calculated (supplemental Table 1) and the above MOIs are used to calculate the daily parasitemia until day 4PI (Figure 1C). Because the period of platelet killing ends on day 4PI, the MOI for each platelet killing scenario now equals 2.8 (0% killing), which is used to calculate the subsequent daily parasitemia until peak (Figure 1C).
With marked iRBC sequestration, parasitemia does not reflect the total parasite burden because sequestered iRBCs are not circulating and counted. If we assume similar levels of sequestration throughout ascending parasitemia, then the slope of log(%parasitemia) between day 0 and 4PI provides the “effective” rate of parasite replication in blood with sequestration and platelet killing. The slope was measured at 0.45, so the “effective MOI” during platelet killing is 100.45 = 2.8. The 80%, 40%, or 0% iRBC killing by platelets has stopped from day 4-7PI, so the “effective” MOI is now: 14, 7, and 2.8, respectively. The calculated parasitemia (Figure 1D) then results in markedly different parasitemia, with 40% and 80% killing by platelets compared with 0% (which fits the observed parasitemia). The slope of log(%parasitemia) did not change markedly after day 4PI as predicted by modeling of parasitemia with sequestration, indicating that platelet killing of iRBCs is minimal, if any.
Platelet inhibition by aspirin has no detectable effect on parasite replication in blood.
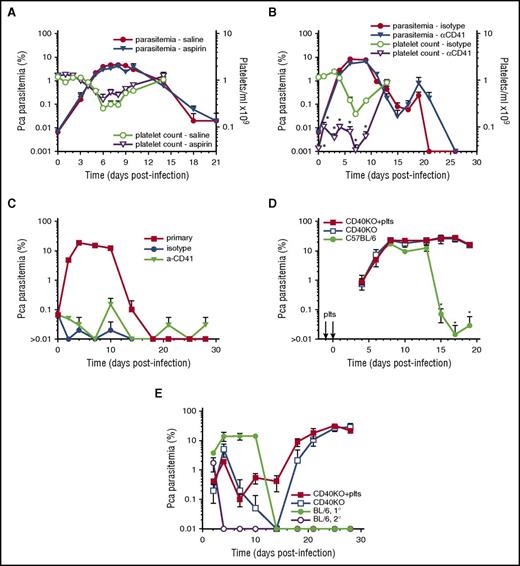
To determine whether inhibition of platelet activation increases parasite replication, we injected high-dose aspirin or vehicle control starting on day-1PI and ending at peak parasitemia (day 10PI); this aspirin regimen was chosen because it was used for the conclusion that aspirin is detrimental in malaria patients based on this aspirin regimen’s elicitation of mortality in Pca DS strain.4,6 Ascending parasitemia was similar in high-dose aspirin and vehicle control mice (Figure 2A). The slope of log(%parasitemia) during ascending parasitemia (day 0-6PI) was similar (P = .49) in both aspirin-treated (0.45 ± 0.03; R2 = 0.99) and vehicle control mice (0.48 ± 0.03; R2 = 0.99). No mortality was observed in either group. Aspirin did not prevent thrombocytopenia during the course of Pca LV infection (Figure 2A).
Platelet removal or inhibition does not affect parasitemia in resolving, non-eCM Pca LV infection and does not affect activation of protective immunity. Parasitemia for each group denoted by filled symbols (left y-axis) with corresponding platelet counts/mL in open symbols (right y-axis) (A-B). (A) Aspirin- (triangle) and saline-injected (circle) groups (n = 5) of Pca-infected mice. (B) Platelet-depleting anti-CD41 mAb (triangle) or isotype control (circle) injected on days –1, 1, 3, and 6PI. (C) Secondary parasitemia after injection of 1 × 107Pca on day 0PI into the anti-CD41 mAb and isotype control groups of mice that had resolved their primary infection; a group of uninfected mice with primary parasitemia is infection control. (D) Primary parasitemia in CD40KO mice that do not resolve Pca infection after IV injection of WT platelets on day −1 and 0PI and in intact mice that resolve their Pca infection. (E) Secondary parasitemia after injection of 1 × 107Pca into 3 groups of mice in panel D; a group of uninfected mice with primary parasitemia is infection control. Average value ± standard error of the mean (SEM) are reported. *P < .05. The experiment in panel A was repeated twice, and in panel B was repeated once.
Platelet removal or inhibition does not affect parasitemia in resolving, non-eCM Pca LV infection and does not affect activation of protective immunity. Parasitemia for each group denoted by filled symbols (left y-axis) with corresponding platelet counts/mL in open symbols (right y-axis) (A-B). (A) Aspirin- (triangle) and saline-injected (circle) groups (n = 5) of Pca-infected mice. (B) Platelet-depleting anti-CD41 mAb (triangle) or isotype control (circle) injected on days –1, 1, 3, and 6PI. (C) Secondary parasitemia after injection of 1 × 107Pca on day 0PI into the anti-CD41 mAb and isotype control groups of mice that had resolved their primary infection; a group of uninfected mice with primary parasitemia is infection control. (D) Primary parasitemia in CD40KO mice that do not resolve Pca infection after IV injection of WT platelets on day −1 and 0PI and in intact mice that resolve their Pca infection. (E) Secondary parasitemia after injection of 1 × 107Pca into 3 groups of mice in panel D; a group of uninfected mice with primary parasitemia is infection control. Average value ± standard error of the mean (SEM) are reported. *P < .05. The experiment in panel A was repeated twice, and in panel B was repeated once.
Platelet depletion exhibits no detectable effect on parasite replication in blood.
To determine whether depletion of platelets early in infection decreases early killing of iRBCs and/or affects the protective immune response, we injected C57BL/6 mice on day –1PI and every 2 to 3 days with either anti-CD41 or isotype control mAb. Few platelets were detected by flow cytometry during the course of Pca infection in platelet-depleted mice during ascending parasitemia, whereas larger numbers of platelets were detected in isotype controls (Figure 2B). Despite >92% platelet depletion from day 0 to 4, there was no significant (P > .05) difference in parasitemia between platelet-depleted and control groups during the course of ascending or descending parasitemia and suppression of the infection (Figure 2B). No mortality was observed in either group.
To determine whether the platelet depletion affects protective immune responses after a second infection, we treated the 2 infected groups, with trimethoprim-sulfamethoxazole for 1 week starting on day 32PI and allowed 1 week for drug clearance before injecting them and an uninfected group IV with 1 × 107Pca iRBCs. The secondary parasitemia was similar and below 0.1% in anti-CD41 and isotype control–injected groups, whereas the primary infection controls parasitemia peaked at 14 ± 1% on day 10PI (Figure 2C). A maximum of 1000 RBCs were counted, resulting in a lower detection limit of 0.1% parasitemia. At least 10 pRBCs/1000 RBCs or 1% parasitemia likely provides a reliable estimate of parasitemia. Most animals exhibited 0 pRBCs/1000 RBCs, resulting in an average parasitemia <0.1%. Removal of platelets by antibody depletion does not affect parasitemia at any point during primary or secondary infections, indicating that platelets are not killing parasites and are not required for activation of the protective immune response.
Intact platelets adoptively transferred to CD40KO recipients do not affect parasitemia or elicit a protective immune response.
To determine whether WT CD40+ platelets adoptively transferred to CD40KO mice (which do not control Pca parasite replication) elicit a reduction in the initial inoculum and/or activate a protective adaptive immune response, we injected CD40-KO mice with 0.2 × 109 platelets from uninfected C57BL/6 mice or saline alone on day –1 and 0PI and infected both groups plus infection control mice group (C57BL/6) with Pca. All groups of mice exhibited similar ascending parasitemia; both groups of CD40KO mice exhibited similarly high levels of unremitting parasitemia, whereas the C57BL/6 mice controlled their parasitemia to low levels (Figure 2D).
We tested the secondary protective immune response in the CD40-KO mice receiving (1) platelets or (2) saline and in C57BL/6 mice by drug curing the 3 groups of animals and then infecting them plus an infection control group with Pca. The secondary parasitemia was similar in both CD40KO groups. Both groups exhibited some initial control and then high levels of parasitemia (Figure 2E). CD40-KO mice develop antiparasite IgM but do not isotype switch to IgG (unpublished results), which may account for the initial control of parasitemia in these mice.
Increased platelet binding to iRBCs and TUNEL+ platelets is not detected in thin blood films during ascending Pca.
To analyze direct platelet cytotoxicity during ascending Pca parasitemia,4,5 we counted apoptotic/dying parasites and iRBC-platelet conjugates in thin blood films. The platelet to target ratio was 1070 ± 55:1 and declined rapidly to about 1 as parasites replicated exponentially during ascending parasitemia and circulating platelet numbers declined (Figure 3A). During ascending parasitemia (day 0-7PI), the %parasitemia using all RBCs is similar to that seen in conjugates of platelets and iRBCs (Figure 3B). Most platelet:RBC conjugates comprise platelets with uRBCs rather than iRBCs (Figure 3C). Less than 10% of iRBCs exhibit an attached platelet and this percentage does not change markedly during the course of Pca infection (Figure 3D). The %iRBCs that are TUNEL+ is significantly greater during peak and descending parasitemia, and most apoptotic parasites do not have a platelet attached to the iRBC (Figure 3E-F). Collectively, these findings indicate that platelet:RBC adhesion is low and few if any TUNEL+ Pca parasites exhibit a bound platelet, indicating that direct platelet adhesion is unlikely to kill significant numbers of parasites.
Platelets are not associated with dying parasites during resolving non-eCM Pca infection in thin blood films. The analyses were performed in thin blood films fluorescence labeled with anti-CD41 (blue; platelets), ethidium bromide (red: parasites), and TUNEL (green). (A) Platelet effector-to-target (iRBC) ratios (filled blue square), platelet:uRBC ratios (open green square), and parasitemia (filled red circle). Platelet:RBC indicates a platelet RBC conjugate; the RBC maybe uninfected (uRBC) or infected (iRBC). (B) Percent PbA parasitemia for all RBCs (red square) ([#iRBC/#RBC]%) and considering only RBCs with bound platelets (blue square) ([#platelet:iRBC/#platelet:RBC]%). (C) Percentage of RBCs with bound platelets with uninfected (red) ([#platelet:uRBC/#platelet:RBC]%) or infected (blue bars) RBCs: ([#platelet:iRBC/#platelet:RBC]%). (D) The percentage of iRBCs with an adherent platelet ([#platelet:iRBC/#iRBC]%). (E) Representative thin blood film made on day 6PI and fluorescently labeled; blue arrows indicate platelets, red arrows indicate parasites without TUNEL, and yellow arrows indicate parasites with TUNEL (green+red). (F) Percentage on days 6, 8, and 12PI of iRBCs exhibiting TUNEL+ labeling (green bar, x-axis) ([(#TUNEL + iRBCs)/#iRBCs]%) and TUNEL– (red bar, x-axis) ([(#TUNEL − iRBCs)/#iRBCs]%) with the percentage of each iRBC+, TUNEL+ or iRBC+, TUNEL– ([(#platelet:TUNEL − iRBCs)/#iRBCs]%) exhibiting an adherent platelet shown as an inset (blue bar). This experiment was repeated twice (n = 5) and verified by flow cytometry. Values are average ± SEM.
Platelets are not associated with dying parasites during resolving non-eCM Pca infection in thin blood films. The analyses were performed in thin blood films fluorescence labeled with anti-CD41 (blue; platelets), ethidium bromide (red: parasites), and TUNEL (green). (A) Platelet effector-to-target (iRBC) ratios (filled blue square), platelet:uRBC ratios (open green square), and parasitemia (filled red circle). Platelet:RBC indicates a platelet RBC conjugate; the RBC maybe uninfected (uRBC) or infected (iRBC). (B) Percent PbA parasitemia for all RBCs (red square) ([#iRBC/#RBC]%) and considering only RBCs with bound platelets (blue square) ([#platelet:iRBC/#platelet:RBC]%). (C) Percentage of RBCs with bound platelets with uninfected (red) ([#platelet:uRBC/#platelet:RBC]%) or infected (blue bars) RBCs: ([#platelet:iRBC/#platelet:RBC]%). (D) The percentage of iRBCs with an adherent platelet ([#platelet:iRBC/#iRBC]%). (E) Representative thin blood film made on day 6PI and fluorescently labeled; blue arrows indicate platelets, red arrows indicate parasites without TUNEL, and yellow arrows indicate parasites with TUNEL (green+red). (F) Percentage on days 6, 8, and 12PI of iRBCs exhibiting TUNEL+ labeling (green bar, x-axis) ([(#TUNEL + iRBCs)/#iRBCs]%) and TUNEL– (red bar, x-axis) ([(#TUNEL − iRBCs)/#iRBCs]%) with the percentage of each iRBC+, TUNEL+ or iRBC+, TUNEL– ([(#platelet:TUNEL − iRBCs)/#iRBCs]%) exhibiting an adherent platelet shown as an inset (blue bar). This experiment was repeated twice (n = 5) and verified by flow cytometry. Values are average ± SEM.
Neither platelet inhibition by aspirin nor platelet depletion affects the replication of HV Pca parasites.
To increase the likelihood of detecting platelet-killing of iRBCs, we increased the initial platelet to iRBC target ratio by 100-fold and increased the time for platelets to kill parasites by injecting 100-fold fewer Pca HV parasites into mice. We injected Pca HV-infected mice with (1) high-dose aspirin, (2) saline, (3) anti-CD41 mAb, or (4) isotype control until peak parasitemia to detect changes in platelet killing of iRBCs and changes in immune response controlling parasite replication. The parasitemia was similar at each time point during ascending parasitemia in aspirin-treated and vehicle controls (supplemental Figure 1A) and anti-CD41 mAb-injected and isotype controls (supplemental Figure 1E). No mortality was observed in either aspirin or vehicle control group. Mortality was similar in anti-CD41 mAb–injected and controls and occurred after ascending parasitemia (supplemental Figure 1E). The parasitemia in RBC-platelet conjugates was greater than that in all RBCs (supplemental Figure 1B) but aspirin did not inhibit this. The percentage of platelet:RBC conjugates was low (<0.1% of RBCs). The number of iRBC-platelet conjugates was low from day 4 to day 8PI and similar to background (day 0PI), but the number of uRBC-platelet conjugates declined (supplemental Figure 1C). The percentage of iRBCs with a bound platelet was <10% throughout the course of infection (supplemental Figure 1D). These findings indicate that neither platelet inhibition by aspirin nor platelet depletion affects parasite replication or the activation of the protective immune response that controls parasitemia.
Human platelets do not inhibit in vitro replication of several strains of Pf.
To confirm that human platelets do not kill Pf-iRBCs in vitro at physiologic concentrations, we incubated platelets with RBCs at a 1:9 ratio with time 0 parasitemia of Pf at trophozoite stage ranging from 4% to 8%. Parasitemia was similar over an entire cycle between cultures containing platelets and those without (Figure 4A). The percent decline in parasitemia in platelet-containing cultures to no-platelet controls was minimal; at 24 and 48 hours, the parasitemia was higher in platelet-containing cultures, providing a negative percent decline (Figure 4B). One donor was tested against FCR3 and DD4 strains of Pf; the parasitemia was similar at each time point in platelet-containing cultures to no-platelet controls. After excluding coincident events, the percentage of iRBCs with bound platelet remained constant and <10%; the percentage of uRBCs with bound platelets was lower and <3% (Figure 4C). Platelets in the cocultures were primarily (∼90%) unbound (Figure 4D). Platelets cultured in media alone exhibited similar thrombin dose responses at early time points, but impaired response at 48 hours (Figure 4E-F).
Human platelets at physiologic ratios do not inhibit in vitro replication of human strains of P falciparum. (A) Representative percent parasitemia measured by imaging flow cytometry over the course of 48 hours in cultures with platelets (open blue square) at 1:9 RBC ratio and without platelets (filled red circle). (B) Calculated replication inhibition of P falciparum replication in platelet cultures compared with no platelet controls (n = 6) at selected time points during the development of P falciparum parasites. (C) The percentage of uRBCs (red bars) ([#platelet:uRBC/#uRBCs]%) or iRBCs (blue bars) ([#platelet:iRBC/#iRBCs]%) with attached platelet over course of P falciparum development. (D) Percentage of all platelets that are unbound (green bars) ([#unboundplatelets/#platelets]%), bound to uRBCs (red bars) ([#platelet:uRBC/#platelets]%), or bound to iRBCs (blue bars) ([#platelet:iRBC/#platelets]%) during the course of P falciparum development. (E) Intensity of CD62P fluorescence on the surface of platelets after stimulation with selected doses of thrombin at selected time points of culture. (F) Calculated thrombin EC50 for platelet response at the selected time points of culture.
Human platelets at physiologic ratios do not inhibit in vitro replication of human strains of P falciparum. (A) Representative percent parasitemia measured by imaging flow cytometry over the course of 48 hours in cultures with platelets (open blue square) at 1:9 RBC ratio and without platelets (filled red circle). (B) Calculated replication inhibition of P falciparum replication in platelet cultures compared with no platelet controls (n = 6) at selected time points during the development of P falciparum parasites. (C) The percentage of uRBCs (red bars) ([#platelet:uRBC/#uRBCs]%) or iRBCs (blue bars) ([#platelet:iRBC/#iRBCs]%) with attached platelet over course of P falciparum development. (D) Percentage of all platelets that are unbound (green bars) ([#unboundplatelets/#platelets]%), bound to uRBCs (red bars) ([#platelet:uRBC/#platelets]%), or bound to iRBCs (blue bars) ([#platelet:iRBC/#platelets]%) during the course of P falciparum development. (E) Intensity of CD62P fluorescence on the surface of platelets after stimulation with selected doses of thrombin at selected time points of culture. (F) Calculated thrombin EC50 for platelet response at the selected time points of culture.
Platelet function in experimental cerebral malaria
Because Pf that elicits human CM is proposed to be susceptible to platelet killing,3,4 the eCM-eliciting parasite, PbA, should also be susceptible to platelet killing. However, this appears to contradict our earlier report that platelet depletion actually protects against eCM without affecting parasitemia.7,8 We therefore reexamined platelet killing in PbA infections of mice.
Neither increased platelet binding to iRBCs and dying (TUNEL+) platelets nor activated, circulating platelets are detected during eCM.
To determine whether platelets function in parasite killing when eCM is elicited, we repeated the Pca analysis with PbA. PbA parasitemia increased exponentially up to and including day 5 (R2 = 0.99 with slope of 0.66) when the rate of increase slows (Figure 5A). Thrombocytopenia starts on day 4, is marked by day 5 (Figure 5A), and occurs during the period of exponential parasite replication. PbA parasitemia in RBCs with bound platelets is significantly higher than all RBCs (P < .05) on day 6PI (Figure 5B). The percentage of platelet-bound iRBCs increased significantly (P < .05) on day 6PI (Figure 5C). However, the percentage of iRBCs at risk for platelet killing declined from day 4 to day 6PI and was <10% (Figure 5D), with no preferential binding of platelets to apoptotic parasites (Figure 5E-F). Platelet depletion by anti-CD41 mAb does not significantly alter PbA parasitemia.7,8
Platelets are not associated with dying parasites during nonresolving, eCM-eliciting PbA in C57BL/6 mice. The analyses were performed in thin blood films fluorescence labeled with anti-CD41 (blue: platelets) and ethidium bromide (red: parasites) and TUNEL (green). (A) Platelet effector-to-target (iRBC) ratios (filled blue square), platelet:uRBC (open green square) ratios, and parasitemia (filled red circle) on left axis and platelet counts/mL (open blue circle) on right axis. (B) Percent PbA parasitemia for all RBCs (red bars) ([#iRBC/#RBC]%) and considering only RBCs with bound platelets (blue bars) ([#platelet:iRBC/#plateletRBC]%). Platelet:RBC indicates a platelet RBC conjugate; the RBC maybe uninfected (uRBC) or infected (iRBC). (C) Percentage of RBCs with bound platelets with uninfected (red bars) ([#platelet:uRBC/#plateletRBC]%) or infected (blue bars) RBCs: ([#platelet:iRBC/#platelet:RBC]%). (D) The percentage of iRBCs with an adherent platelet ([#platelet:iRBC/#iRBCs]%). (E) Representative thin blood film made on day 6PI and fluorescently labeled; blue arrows indicate platelets, red arrows indicate parasites without TUNEL, and yellow arrows indicate parasites with TUNEL (green+red). (F) Percentage on days 4 and 6PI of iRBCs exhibiting TUNEL+ labeling (red bar, x-axis) ([(#TUNEL + iRBCs)/#iRBCs]%) and TUNEL– (green bar, x-axis) ([(#TUNEL − iRBCs)/#iRBCs]%) with the percentage of each iRBC+, TUNEL+ or iRBC+, TUNEL– ([(#platelet:TUNEL − iRBCs)/#iRBCs]%) exhibiting an adherent platelet shown as an inset (blue bar). This experiment was repeated twice (n = 5) and verified by flow cytometry. Values are average ± SEM.
Platelets are not associated with dying parasites during nonresolving, eCM-eliciting PbA in C57BL/6 mice. The analyses were performed in thin blood films fluorescence labeled with anti-CD41 (blue: platelets) and ethidium bromide (red: parasites) and TUNEL (green). (A) Platelet effector-to-target (iRBC) ratios (filled blue square), platelet:uRBC (open green square) ratios, and parasitemia (filled red circle) on left axis and platelet counts/mL (open blue circle) on right axis. (B) Percent PbA parasitemia for all RBCs (red bars) ([#iRBC/#RBC]%) and considering only RBCs with bound platelets (blue bars) ([#platelet:iRBC/#plateletRBC]%). Platelet:RBC indicates a platelet RBC conjugate; the RBC maybe uninfected (uRBC) or infected (iRBC). (C) Percentage of RBCs with bound platelets with uninfected (red bars) ([#platelet:uRBC/#plateletRBC]%) or infected (blue bars) RBCs: ([#platelet:iRBC/#platelet:RBC]%). (D) The percentage of iRBCs with an adherent platelet ([#platelet:iRBC/#iRBCs]%). (E) Representative thin blood film made on day 6PI and fluorescently labeled; blue arrows indicate platelets, red arrows indicate parasites without TUNEL, and yellow arrows indicate parasites with TUNEL (green+red). (F) Percentage on days 4 and 6PI of iRBCs exhibiting TUNEL+ labeling (red bar, x-axis) ([(#TUNEL + iRBCs)/#iRBCs]%) and TUNEL– (green bar, x-axis) ([(#TUNEL − iRBCs)/#iRBCs]%) with the percentage of each iRBC+, TUNEL+ or iRBC+, TUNEL– ([(#platelet:TUNEL − iRBCs)/#iRBCs]%) exhibiting an adherent platelet shown as an inset (blue bar). This experiment was repeated twice (n = 5) and verified by flow cytometry. Values are average ± SEM.
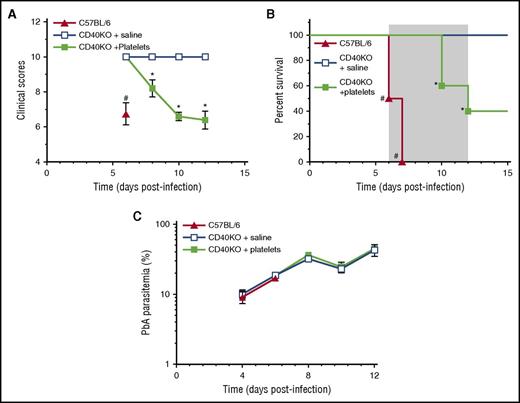
To determine whether WT platelets transferred to CD40KO mice, which are eCM resistant,19 affects parasitemia and/or elicits a pathogenic response, we injected 0.2 × 109 platelets from uninfected mice or vehicle control into eCM-protected CD40-KO mice19 on day –1 and day 1PI. Significantly (P < .05) decreased clinical scores and increased mortality in CD40-KO mice receiving platelets compared with vehicle controls (Figure 6A-B) indicate that CD40+ platelets elicit pathogenesis in a resistant CD40KO mouse; consequently, platelet CD40 is a key molecule in triggering pathogenesis and platelets do not affect PbA replication in vivo (Figure 6C). Together with Pca findings, they also indicate that the pathways leading to control of parasite replication and pathogenesis are separate and distinct.
Platelets partially restore pathogenesis to eCM-protected CD40-KO mice. (A) Clinical scores, (B) percent survival, and (C) parasitemia for groups (n = 5) CD40-KO reconstituted with WT platelets (filled green square), CD40-KO injected with saline (open blue square), or C57BL/6 controls (filled red triangle). Gray shaded area indicates the time period for the development of neurologic symptoms by C57BL/6 mice. Values are average ± SEM. *P < .05 for comparison of CD40-KO + platelets groups with other 2 groups; #P < .05 for comparison of C57BL/6 with other 2 groups.
Platelets partially restore pathogenesis to eCM-protected CD40-KO mice. (A) Clinical scores, (B) percent survival, and (C) parasitemia for groups (n = 5) CD40-KO reconstituted with WT platelets (filled green square), CD40-KO injected with saline (open blue square), or C57BL/6 controls (filled red triangle). Gray shaded area indicates the time period for the development of neurologic symptoms by C57BL/6 mice. Values are average ± SEM. *P < .05 for comparison of CD40-KO + platelets groups with other 2 groups; #P < .05 for comparison of C57BL/6 with other 2 groups.
Discussion
The explanations for thrombocytopenia correlating with severe malaria include (1) platelets kill iRBCs, (2) platelets activate the protective immune response, and/or (3) absence of platelets contributes to pathogenesis. As a consequence of (1) and (2), thrombocytopenia would result in increased parasitemia and disease. Two groups3-5 have proposed platelet killing of Plasmodium in iRBCs. The first line of evidence supporting platelet killing of iRBCs is the concurrence of thrombocytopenia during Pca infection in mice with the development of patent parasitemia and the exponential rise in parasitemia until peak parasitemia.4 However, the parasite is replicating exponentially in the blood compartment from the time of initial infection to about peak parasitemia; thus, the log(%parasitemia) fits with a straight line. Our data indicate that the slope of this line does not change with the onset of thrombocytopenia, which is predicted if platelets are controlling parasite replication. Thus, the coincidence of patent parasitemia and thrombocytopenia is likely a result of the host response the parasite has elicited rather than the direct killing of iRBCs by platelets.
Second, depletion of platelets by mAb leads to increased parasitemia in PbA-infected mice.20 Significantly increased parasitemia occurs at a single time point (day 5PI) in PbA-infected mice depleted of platelets with anti-CD42b mAb on day −1PI compared with isotype Ig controls.20 Parasitemia measured at a single time point by conventional flow cytometry may yield incorrect results because some uRBCs contain nucleic acids that are then interpreted as iRBCs. In the present study, we used a different mAb to deplete platelets (anti-CD41 mAb MWreg40) and verified our depletion throughout ascending parasitemia, measured parasitemia at multiple time points, and tested 2 different species of Plasmodium without detecting any effect of platelet depletion.7,8 Both McMorran’s4 and our analyses of inhibition of platelet activity by high-dose aspirin indicate that this regimen does not affect Pca or PbA parasitemia. Our data therefore indicate that neither inhibition of platelets nor depletion of platelets with mAb affects parasitemia.
McMorran et al4 use death rather than parasitemia to detect platelet killing of parasites because iRBC sequestration in organs may result in parasitemia not reflecting total numbers of parasites, resulting in an inability to detect parasite killing by platelets. However, our data (Figure 1) indicate that the slope of log(%parasitemia) during ascending parasitemia is linear and dependent on parasite virulence. Parasitemia and the slope of log(%parasitemia), therefore, should be different in the presence and absence of platelets given the 80% killing observed in vitro.4,5
The use of the animal’s death as a biomarker for parasite replication maybe problematic. Death is complex, multifactorial, and occurs at low and high parasitemia, so death may not be directly linked to parasite burden. Indeed, the majority of deaths reported by McMorran et al4 occur after ascending parasitemia at time points when parasite burden is being suppressed by the adaptive immunity11,17,18 and thrombocytopenia has been marked for a while. Thus, there is little evidence to indicate that an elevated parasite burden is the cause of the animal’s death.
The platelet-mediated killing of iRBCs in vivo is proposed to be contact-dependent based on: (1) increased Pca parasitemia when platelet-RBC conjugates are counted compared with all RBCs and (2) significantly (P < .05) increased percentage of parasites that are TUNEL+ in platelet-deficient MPL−/− mice compared with C57BL/6 controls.4 However, our analysis of platelet:xRBC (x = u or i) conjugates during the course of Pca infection supports the conclusion that platelets are not cytotoxic for parasites. Our findings that most platelets adhere to uRBCs, <10% of iRBCs have a bound platelet, and few apoptotic parasites occur in platelet:iRBC conjugates, collectively indicate that it is unlikely that platelets are adhering to iRBCs early during the infection before the onset of thrombocytopenia to kill the parasite within the RBC.
Others reported contact-dependent intra-Pf-RBC killing by human platelets in vitro.3-5 Peyron et al5 reported that a platelet:Pf-iRBC ratio of 160:1 (corresponding to a platelet:RBC ratio ∼1:1, which is not physiologic) results in >80% inhibition of parasite development. If this finding is valid, then platelet inhibition should reduce this killing. However, in this study, in vitro platelet killing of Pf-iRBC is not inhibited by 1 mM aspirin or 2 mM histidine. In contrast, McMorran et al3,4 reported that this platelet killing is abrogated by known platelet inhibitors. However, their observation that the total parasitemia at 29 hours (trophozoite) and 44 hours (schizont) of platelet-Pf-iRBC cocultures are similar suggests that 15 hours of culture have passed with no killing or inhibition of development detected in the presence of platelets.4 In the present study, we also did not detect any change in parasitemia with Pf ring-stages maturing to schizonts in the presence or absence of platelets. We replicated our in vivo condition as much as possible with human cells at 1:9 platelet:RBC ratio of normal blood and ∼2:1 platelet:iRBC ratio. We tested multiple human donors and parasite strains, confirmed platelet thrombin responses throughout the coculture, but did not detect inhibition of parasite replication or development by platelets. The percentage of platelets bound to a Pf-iRBC was greater than uRBC, but these percentages were <10% and remained constant throughout the coculture. Clinical studies report either an association of elevated numbers of platelet-RBCs conjugates (rosettes) with severe human malaria or no association.1,2 Platelet-Pf-iRBC rosettes associate with high parasitemia and cerebral malaria.21 If platelets killed Pf-iRBCs within rosettes, then elevated rosette numbers should reduce parasitemia and be associated with lower parasitemia and likely with protection from severe malaria. Collectively, these observations suggest that under physiologic conditions, platelets do not inhibit the replication or intra-RBC development of Pf. Thus, there is no rationale for avoiding the use of nonsteroidal anti-inflammatory drugs in the treatment of malaria.6
Besides their putative role as innate killer cells, platelets function in the activation of immune responses. Indeed, CD40L+ WT platelets adoptively transferred to CD40LΚΟ mice activate the protective immune response in virus- or bacteria-infected mice.22-24 However, neither high-dose aspirin nor platelet depletion starting at day −1PI affected the primary parasitemia. Platelet depletion during the primary infection also did not alter the secondary parasitemia. Moreover, adoptive transfer of CD40+ WT platelets into CD40ΚΟ mice that do not control their Pca parasitemia had no detectable effect on the primary and secondary immune responses controlling parasitemia. These results indicate that (1) adoptive transfer of WT CD40+ platelets to CD40KO mice does not restore immune control of primary or secondary parasitemias, as reported in some other infections,22-24 and (2) platelets are not required for the development of a protective primary and secondary immune responses to resolve Pca.
The observations that C57BL/6 mice depleted of platelets by anti-CD41 mAb injection early (day 1PI) but not late (day 4 onwards) exhibit decreased inflammatory responses, decreased acute-phase proteins, and are protected from eCM7,8 indicate that the activation of the pathogenic eCM response is impaired in the absence of platelets. Their requirement for the onset of the pathogenic response has however not been tested. Our observation that adoptive transfer of WT CD40+ platelets to eCM-resistant CD40ΚΟ mice partially restores mortality, and clinical symptoms of eCM supports the role of platelet CD40 in activating a pathogenic response.
Collectively, our findings indicate that platelets (1) do not function in the killing of blood-stage Plasmodium parasite, (2) are not required for the activation of the protective immune response, but (3) are required to elicit pathogenesis. Platelets may function in part by activating the liver to produce acute-phase proteins or by activating the pathogenic immune response.8,20
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health, National Institute of Allergy and Infectious Diseases grant R03AI088283, and National Institutes of Health, National Institute of Neurological Disorders and Stroke grants R21NS066401 (H.C.v.d.H.), R03NS081527, 5R21NS080063 (I.G.), and R01NS079873 (V.C.).
Authorship
Contribution: I.G. and H.C.v.d.H. developed hypotheses, designed and performed the experiments, analyzed data, and wrote the manuscript; J.V. and M.W. performed experiments and analyzed data; and V.C. and G.E.R.G. developed hypotheses, reviewed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Irene Gramaglia, La Jolla Infectious Disease Institute, 3525 Del Mar Heights Rd #453, San Diego, CA 92130; e-mail: igramaglia@ljidi.org.

![Figure 3. Platelets are not associated with dying parasites during resolving non-eCM Pca infection in thin blood films. The analyses were performed in thin blood films fluorescence labeled with anti-CD41 (blue; platelets), ethidium bromide (red: parasites), and TUNEL (green). (A) Platelet effector-to-target (iRBC) ratios (filled blue square), platelet:uRBC ratios (open green square), and parasitemia (filled red circle). Platelet:RBC indicates a platelet RBC conjugate; the RBC maybe uninfected (uRBC) or infected (iRBC). (B) Percent PbA parasitemia for all RBCs (red square) ([#iRBC/#RBC]%) and considering only RBCs with bound platelets (blue square) ([#platelet:iRBC/#platelet:RBC]%). (C) Percentage of RBCs with bound platelets with uninfected (red) ([#platelet:uRBC/#platelet:RBC]%) or infected (blue bars) RBCs: ([#platelet:iRBC/#platelet:RBC]%). (D) The percentage of iRBCs with an adherent platelet ([#platelet:iRBC/#iRBC]%). (E) Representative thin blood film made on day 6PI and fluorescently labeled; blue arrows indicate platelets, red arrows indicate parasites without TUNEL, and yellow arrows indicate parasites with TUNEL (green+red). (F) Percentage on days 6, 8, and 12PI of iRBCs exhibiting TUNEL+ labeling (green bar, x-axis) ([(#TUNEL + iRBCs)/#iRBCs]%) and TUNEL– (red bar, x-axis) ([(#TUNEL − iRBCs)/#iRBCs]%) with the percentage of each iRBC+, TUNEL+ or iRBC+, TUNEL– ([(#platelet:TUNEL − iRBCs)/#iRBCs]%) exhibiting an adherent platelet shown as an inset (blue bar). This experiment was repeated twice (n = 5) and verified by flow cytometry. Values are average ± SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/12/10.1182_blood-2016-08-733519/4/m_blood733519f3.jpeg?Expires=1769321440&Signature=v9HXlQbd3GkeFqio2NPbs1~molhvzxMnzpX19HKcD~aO2cNi4wjvMdOobgmyb0o6921vPF38c4QO87hJfD5klO3qt5Rv6VpY0uYtwmTMOOzDzHHo1DOwgS-snICyBjvLo1kITbmqToITNBwfSx94nNVfdiX5o97So7d10cg5ULxJYJftINXAT0ed0hzwKyZij17j9N1GnmjZL8LWo7XN1~eMcdCjUgQFXG6ijVczFOiTToMsdY9AWxEpBEYgNS4KhxVhqWLoKyOgEzgwcFFws4n9iz7Nm-a8UJ-hW3sT6mWxEI40TOrpKcJY3na194tsjCf2GTdkRmC8XR0MBwubwg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Human platelets at physiologic ratios do not inhibit in vitro replication of human strains of P falciparum. (A) Representative percent parasitemia measured by imaging flow cytometry over the course of 48 hours in cultures with platelets (open blue square) at 1:9 RBC ratio and without platelets (filled red circle). (B) Calculated replication inhibition of P falciparum replication in platelet cultures compared with no platelet controls (n = 6) at selected time points during the development of P falciparum parasites. (C) The percentage of uRBCs (red bars) ([#platelet:uRBC/#uRBCs]%) or iRBCs (blue bars) ([#platelet:iRBC/#iRBCs]%) with attached platelet over course of P falciparum development. (D) Percentage of all platelets that are unbound (green bars) ([#unboundplatelets/#platelets]%), bound to uRBCs (red bars) ([#platelet:uRBC/#platelets]%), or bound to iRBCs (blue bars) ([#platelet:iRBC/#platelets]%) during the course of P falciparum development. (E) Intensity of CD62P fluorescence on the surface of platelets after stimulation with selected doses of thrombin at selected time points of culture. (F) Calculated thrombin EC50 for platelet response at the selected time points of culture.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/12/10.1182_blood-2016-08-733519/4/m_blood733519f4.jpeg?Expires=1769321440&Signature=Z6ExkqUtgG4LrYdbQZ8I8oCnOqW8OGxw3C2mIbNwWq2drPlfqDDKKjjw0Gl8RA31NHiRRaRu72m6l~-2PM7pOOoj15FZagRih6hSxyOysOHBXsoBYx6pDO5dLdEuyq-d8PEpKmqakXL8OPa6-HonJAdG91FSRsKCTx0UMOutszTr2T~gc3z9zbzrwDbNB7zl6Yb5YycJeS2fzfcFs25sjZENlVBe~LZOL~QIROs5w7Yi8ByrjLEw8K0GV241yysitEsF-XrTkr1SXKKSsz8GN6Wf7wKAGHo2gVVp1VrZJJRE8huuGoi67E7R7ptMNgMumnvFPYYUAN8uHxLqON0nOA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Platelets are not associated with dying parasites during nonresolving, eCM-eliciting PbA in C57BL/6 mice. The analyses were performed in thin blood films fluorescence labeled with anti-CD41 (blue: platelets) and ethidium bromide (red: parasites) and TUNEL (green). (A) Platelet effector-to-target (iRBC) ratios (filled blue square), platelet:uRBC (open green square) ratios, and parasitemia (filled red circle) on left axis and platelet counts/mL (open blue circle) on right axis. (B) Percent PbA parasitemia for all RBCs (red bars) ([#iRBC/#RBC]%) and considering only RBCs with bound platelets (blue bars) ([#platelet:iRBC/#plateletRBC]%). Platelet:RBC indicates a platelet RBC conjugate; the RBC maybe uninfected (uRBC) or infected (iRBC). (C) Percentage of RBCs with bound platelets with uninfected (red bars) ([#platelet:uRBC/#plateletRBC]%) or infected (blue bars) RBCs: ([#platelet:iRBC/#platelet:RBC]%). (D) The percentage of iRBCs with an adherent platelet ([#platelet:iRBC/#iRBCs]%). (E) Representative thin blood film made on day 6PI and fluorescently labeled; blue arrows indicate platelets, red arrows indicate parasites without TUNEL, and yellow arrows indicate parasites with TUNEL (green+red). (F) Percentage on days 4 and 6PI of iRBCs exhibiting TUNEL+ labeling (red bar, x-axis) ([(#TUNEL + iRBCs)/#iRBCs]%) and TUNEL– (green bar, x-axis) ([(#TUNEL − iRBCs)/#iRBCs]%) with the percentage of each iRBC+, TUNEL+ or iRBC+, TUNEL– ([(#platelet:TUNEL − iRBCs)/#iRBCs]%) exhibiting an adherent platelet shown as an inset (blue bar). This experiment was repeated twice (n = 5) and verified by flow cytometry. Values are average ± SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/12/10.1182_blood-2016-08-733519/4/m_blood733519f5.jpeg?Expires=1769321440&Signature=rVUblZSO6Fzqaiu4trVpVYv-RbMv9lfZ8MKYH7jTuIlpOezGQrWvHL1Fop9naWSVwdatTbvX1z0~Io0t4jvNUUVDg4T01hkWIqxIsegIFqWvcw~eecvkiZXCRXeTSs1r2iXzU5cPkzZ2gX-ZpjQg8lWzUARO3NNzPaZE~wYr0Ui0ByTXIpT7b069Zx6Iw3RroeH3gQ6QUtTh7qwGjHOwEsPLddCx3N98hcEVyLxLAfb7nn6ATlw3BFOMoKOaX3hDRxzDHbbneoGuAo2HJJ7ihV7U4BXTJ~NoeHOAFxTdfBhlizw9jR70rqkQhPKkrvxaAjCOouyusOEFY9YA~Ls9tA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)