Key Points
Tolerance after bone marrow transplantation requires CD8+ DCs and NKT-cell interaction.
CD8+ DCs and NKT cells become tolerogenic after conditioning with total lymphoid irradiation.
Abstract
The combination of total lymphoid irradiation and anti-T-cell antibodies safely induces immune tolerance to combined hematopoietic cell and organ allografts in humans. Our mouse model required host natural killer T (NKT) cells to induce tolerance. Because NKT cells normally depend on signals from CD8+ dendritic cells (DCs) for their activation, we used the mouse model to test the hypothesis that, after lymphoid irradiation, host CD8+ DCs play a requisite role in tolerance induction through interactions with NKT cells. Selective deficiency of either CD8+ DCs or NKT cells abrogated chimerism and organ graft acceptance. After radiation, the CD8+ DCs increased expression of surface molecules required for NKT and apoptotic cell interactions and developed suppressive immune functions, including production of indoleamine 2,3-deoxygenase. Injection of naive mice with apoptotic spleen cells generated by irradiation led to DC changes similar to those induced by lymphoid radiation, suggesting that apoptotic body ingestion by CD8+ DCs initiates tolerance induction. Tolerogenic CD8+ DCs induced the development of tolerogenic NKT cells with a marked T helper 2 cell bias that, in turn, regulated the differentiation of the DCs and suppressed rejection of the transplants. Thus, reciprocal interactions between CD8+ DCs and invariant NKT cells are required for tolerance induction in this system that was translated into a successful clinical protocol.
Introduction
Our clinical studies have demonstrated that conditioning patients given combined kidney and hematopoietic cell transplants with total lymphoid irradiation (TLI) and antithymocyte globulin (ATG) reliably induces mixed chimerism and tolerance to the transplanted living donor kidney in HLA-matched patients, enabling complete withdrawal of antirejection medications without severe neutropenia, thrombopenia, graft-versus-host disease or other serious complications.1-3 Recently, we have also achieved persistent mixed chimerism in living donor HLA-haplomatched transplant patients as a stepping stone toward tolerance induction in deceased donor transplant recipients with partial or no HLA matching.3 Given the benefit to patients of approaches that can achieve tolerance to the allograft, a few groups including our own developed clinical protocols based on mouse models that investigated the cellular and molecular requirements for successful tolerance induction.4 Our own work has shown that TLI/ATG conditioning establishes a tolerogenic microenvironment involving a network of adaptive and innate immune-regulatory cells.5-9
We investigated the cellular basis of immune tolerance further in a murine model5-9 of our clinical protocol.1-3 The TLI/ATG conditioning regimen induces rapid and dramatic depletion of naive T cells and other leukocytes via the p53-Bcl2 apoptotic pathway,10 and favors the balance of natural killer T (NKT) cells over naive T cells in both mouse and human recipients.1-11 Tolerance induction in this model system has been shown to be dependent on NKT cells and their secretion of interleukin 4 (IL-4).5-8 However, because NKT cells are highly responsive to signals from other cells, we considered the possibility that the effect of TLI on NKT cells is indirect and that other cell types such as dendritic cells (DCs) that interact with NKT cells will determine the NKT-cell functions. The DCs are not only the most potent antigen-presenting cells in the establishment of T-cell immunity to eliminate viruses and tumors,12,13 but they can also play a critical role in the induction of immune tolerance.14-18 Tolerogenic DCs are characterized by reduced expression of costimulatory molecules such as CD80, CD86, and CD40, loss of antigen-presenting functions, gain of immunosuppressive functions including production of the tryptophan catabolic enzyme, indoleamine 2,3-deoxygenase (IDO), and polarization of cytokine production away from IL-12 and toward IL-10.14-18 Multiple ligand-receptor interactions and signaling pathways influence the decision of DCs to become “tolerogenic” or “immunogenic”. “Danger signals” including nucleotides, proinflammatory cytokines, and selected Toll-like receptor ligands promote an immunogenic phenotype,12,13 whereas interactions with CTLA4 on regulatory T cells (Tregs), stimuli that control interferon production, and uptake of apoptotic bodies or fragmented DNA have been shown to promote a tolerogenic phenotype.19-22
We focused on CD8+ DCs because they are the predominant DC subset that can present a variety of glycolipids in association with CD1d to invariant NKT (iNKT) cells to induce activation via the NKT-cell T-cell receptor (TCR).23 In addition, IDO produced by DCs can polarize NKT cells toward IL-4 secretion.24
Methods
Mice
Adult 8- to 10-week-old male Batf3−/− BALB/c (H-2Kd)25 mice and wild-type C57BL/6 (H-2Kb) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). C57BL/6 neonates were purchased from Charles River Laboratories (Wilmington, MA). Wild-type BALB/c (H-2Kd), Cd1−/− BALB/c mice,26 Ja18−/− BALB/c mice,27 and NKG2D-deficient (Klrk1−/−) C57BL/6 mice28 (provided by Sheri Krams, Department of Surgery, Stanford University) were bred at Stanford University.
Cardiac transplantation and monitoring for graft survival
Neonatal C57BL/6 heart grafts were transplanted into a pouch in the ear pinna of BALB/c hosts on day 0, and were monitored for rejection according to the procedures described previously.29
Bone marrow transplantation, rabbit ATS, and TLI
The bone marrow harvesting and transplantation procedure was performed as previously described.29 BALB/c recipients were injected intraperitoneally (i.p.) with 0.05 mL of rabbit antithymocyte serum (ATS) (Accurate Chemical and Scientific Inc, Westbury, NY) in 0.5 mL of saline on days 0, 2, 6, 8, and 10 after heart transplantation. TLI was delivered to the lymph nodes above and below the diaphragm, thymus, and spleen with lead shielding of the skull, limbs, pelvis, and tail using a 250-Kv x-ray machine.29 Ten doses of 240 cGy each were administered starting on the day of heart transplantation using 5 doses per week.6,7 The last dose of TLI was administered to BALB/c mice 24 hours before the allogeneic bone marrow cell infusions on day 15 after heart transplantation.
Immunofluorescent staining and FACS analysis
Staining procedures and flow cytometry analysis have previously been described in detail.6 Fluorochrome-conjugated monoclonal antibodies (mAbs) used for cell-surface staining were purchased from eBioscience (San Diego, CA), Biolegend (San Diego, CA), BD Biosciences (San Jose, CA), and R&D Systems (Minneapolis, MN). Rabbit anti-IDO antibodies were purchased from Millipore (Temecula, CA), and phycoerythrin-conjugated goat anti-rabbit immunoglobulin G (IgG) antibody from Santa Cruz Biotechnology (Santa Cruz, CA). For intracellular IDO and Foxp3 staining, cells were first stained with aqua dye (Zombie Aqua; Biolegend, San Diego, CA), a dead cell exclusion dye, prior to fixation and permeabilization as per the manufacturer’s protocol (eBioscience/BD Biosciences). Phycoerythrin-conjugated CD1d tetramers were obtained from the National Institutes of Health (NIH) Tetramer Facility (Rockville, MD). Fluorescence-activated cell sorter (FACS) analysis used FlowJo software.
Chimerism analysis
Isolation and purification of CD8α+ DCs and NKT cells
Single spleen cell suspensions were enriched using anti-CD11c (N418) microbeads and magnetic-activated cell sorting columns (Miltenyi Biotec Inc, San Diego, CA). Purified cells were then stained with anti-CD11c, anti- I-E/I-A (major histocompatibility complex II [MHCII]), CD8α, and anti-CD11b mAbs prior to sort on an Aria flow cytometer (Becton Dickinson) to >99% purity. iNKT cells were isolated as described before.30
Isolation of gut DCs
Cells were isolated from the gut as previously described,31 and stained with CD11c vs CD103 and CD8 or CD11b mAbs.
In vitro CD8+ DC suppression assays (mixed leukocyte reactions)
BALB/c responder cells (splenocytes) were labeled with 2.5 μM carboxyfluorescein (CFSE) (Molecular Probes/Invitrogen, Eugene, OR) and C57BL/6 stimulator cells were irradiated (3000 rad) from a 137Cs source (J. L. Shephard & Associates, San Fernando, CA). Subsequently, CFSE-labeled BALB/c responder cells (1 × 105 cells per well), and irradiated C57BL/6 stimulators (2 × 105 cells per well) were cocultured with or without sorted CD8α+ DCs (1 × 105 cells per well) for 5 days, and then analyzed for suppression of proliferation as described previously.7 In some assays, 1-methyl-l-tryptophan (250 μg/mL) was added to cultures.
Coculture of CD8+ DCs and NKT cells and cytokine secretion
Sorted iNKT cells (5 × 104 cells per well) and CD8α+ DCs (1 × 104 cells per well) were cultured at 37°C, 5% CO2 in Complete (10% fetal calf serum) RPMI 1640 medium for 4 days. After culture, the supernatants were harvested, and the concentrations of cytokines in supernatants were quantified using a MAGPIX instrument (Luminex Corporation, TX). In some cultures, 100 ng/mL α-galactocylceramide (α-GalCer) was added.
Results
CD8+ DCs, as well as NKT cells are required for chimerism and tolerance induction following TLI
To assess the role of CD8+ DCs in chimerism, and the induction of tolerance following TLI/ATS conditioning, BALB/c (H-2d) recipient mice were given combined bone marrow and heart transplants from MHC-mismatched C57BL/6 (H-2b) donors. As shown in the experimental scheme in Figure 1A, recipients received an organ allograft on day 0, and conditioning with 10 doses of TLI and 5 doses of ATS during a 15-day period thereafter. Bone marrow cells were infused immediately after the last dose of TLI, and chimerism and graft acceptance were assessed at serial time points thereafter. Figure 1B shows that all wild-type BALB/c hosts accepted the heart allografts for at least 100 days, and none were rejected. In contrast, Jα18−/− BALB/c hosts lacking iNKT cells all rejected their heart grafts by day 30 after transplantation. Similarly, about 85% of Batf3−/− BALB/c hosts lacking CD8+ DCs25 rejected their heart grafts by day 90 (Figure 1B). All recipients that rejected their heart grafts failed to develop chimerism (Figure 1C).
CD8+ DCs, NKT cells, and expression of NKG2D are required for heart allograft survival and development of chimerism after combined BMT and heart transplantation. (A) Experimental scheme: BALB/c or C57BL/6 hosts were given donor C57BL/6 or BALB/c neonatal heart transplants (HTX), respectively, on day 0, and ATS was injected i.p. on days 0, 2, 6, 8, and 10. Hosts were conditioned over 14 days with 10 doses of TLI of 240 cGy each. On day 15, 50 × 106 C57BL/6 or BALB/c donor bone marrow cells from the same strain as the heart grafts were injected IV (bone marrow transplantation [BMT]), and chimerism and heart graft survival were monitored for 100 days after organ transplantation. (B) Percentages of hosts with heart graft survival among wild-type (WT) BALB/c (n = 10), or Batf3−/− (n = 8), or Jα18−/− (n = 8) hosts or WT C57BL/6 (n = 10) or NKG2D-deficient (Klrk1−/−) (n = 8) hosts at serial time points. WT TX (BALB/c) vs Batf3−/− TX: P < .001; WT TX (BALB/c) vs Jα18−/− TX: P < .001; WT TX (B6) vs NKG2D-deficient (Klrk1−/−) TX (B6): P < .001; Batf3−/− TX vs Jα18−/− TX: P < .001 (log-rank; Mantel-Cox test). (C) Percentages of donor type cells among (T cells, B cells, macrophages, and granulocytes in the blood of hosts 28 days after BMT. Bars show mean percentages of donor cells. P values by the 2-tailed t test of independent means. *P < .05; **P < .01; ***P < .001; NS, not significant (P > .05).
CD8+ DCs, NKT cells, and expression of NKG2D are required for heart allograft survival and development of chimerism after combined BMT and heart transplantation. (A) Experimental scheme: BALB/c or C57BL/6 hosts were given donor C57BL/6 or BALB/c neonatal heart transplants (HTX), respectively, on day 0, and ATS was injected i.p. on days 0, 2, 6, 8, and 10. Hosts were conditioned over 14 days with 10 doses of TLI of 240 cGy each. On day 15, 50 × 106 C57BL/6 or BALB/c donor bone marrow cells from the same strain as the heart grafts were injected IV (bone marrow transplantation [BMT]), and chimerism and heart graft survival were monitored for 100 days after organ transplantation. (B) Percentages of hosts with heart graft survival among wild-type (WT) BALB/c (n = 10), or Batf3−/− (n = 8), or Jα18−/− (n = 8) hosts or WT C57BL/6 (n = 10) or NKG2D-deficient (Klrk1−/−) (n = 8) hosts at serial time points. WT TX (BALB/c) vs Batf3−/− TX: P < .001; WT TX (BALB/c) vs Jα18−/− TX: P < .001; WT TX (B6) vs NKG2D-deficient (Klrk1−/−) TX (B6): P < .001; Batf3−/− TX vs Jα18−/− TX: P < .001 (log-rank; Mantel-Cox test). (C) Percentages of donor type cells among (T cells, B cells, macrophages, and granulocytes in the blood of hosts 28 days after BMT. Bars show mean percentages of donor cells. P values by the 2-tailed t test of independent means. *P < .05; **P < .01; ***P < .001; NS, not significant (P > .05).
To examine the possible role of NKG2D, an activation marker that is constitutively expressed on NKT as well as NK cells,32,33 we compared chimerism and tolerance in Klrk1−/− (NKG2D-deficient)28 and wild-type hosts. Because mice with the inactivated gene were available only on the C57BL/6 background, we compared the outcomes using wild-type or NKG2D-deficient mice as hosts, and BALB/c mice as donors using the same procedures as outlined in Figure 1A. As shown in Figure 1B-C, TLI-conditioned hosts lacking NKG2D expression rejected their heart and marrow allografts by 40 days posttransplant, whereas none of the wild-type mice rejected their heart allografts by 100 days posttransplant. Wild-type BALB/c and C57BL/6 recipients had mixed chimerism in all lineages, with the highest chimerism among B cells and lowest among T cells (Figure 1C). The results showed that chimerism and tolerance were abrogated in hosts with CD8+ DC or NKT-cell deficiencies, and suggested that NKG2D on NKT cells is also required.
CD8+ DCs are the predominant DC subset remaining after TLI and their accumulation is enhanced by NKT cells
Three major subsets of CD11c+ DCs (CD11b+CD8−, CD11b−CD8−, and CD11b−CD8+), all of which have been described previously,34 were identified in the spleens of BALB/c mice before and after conditioning with 10 doses of TLI of 240 cGy each (Figure 2A). All of the CD11c+ DCs were MHCIIhi. In wild-type untreated mice, the CD11b−CD8− subset accounted for 67% of DCs and the 2 minor subsets of CD11b−CD8+ and CD11b+CD8− cells accounted for a mean of 25% and 8%, respectively (Figure 2B-D). Five days after TLI, the balance of DC subsets in wild-type mice changed significantly such that the CD11b−CD8+ subsets became predominant (mean about 60%), and CD11b−CD8− cells were reduced to a mean of about 20%. The CD11b+CD8− subset was significantly increased to a mean of about 20% (Figure 2B-D).
TLI conditioning that favors the accumulation of CD8+ DCs is dependent on NKT cells. (A) Representative 2-color FACS patterns of CD8α vs CD11b on gated CD11c+ spleen cells from untreated (UNT) wild-type (n = 6) or Jα18−/− (n = 5) or Batf3−/− (n = 5) or from TLI-conditioned wild-type (n = 11), Batf3−/− (n = 7), or Jα18−/− (n = 8) BALB/c mice, at day 5 after completion of TLI. Percentages of DC subsets in each box are shown. (B) Mean (± standard error of the mean [SEM]) percentages of: CD11c+CD11b−CD8α+ cells (C), CD11c+CD11b+CD8α− cells (D), CD11c+CD11b−CD8α− cells (E). Representative 2-color FACS pattern of 7AAD vs Annexin V staining of spleen cells immediately after completion of 10 doses of TLI in a BALB/c mouse. SSC, side scatter.
TLI conditioning that favors the accumulation of CD8+ DCs is dependent on NKT cells. (A) Representative 2-color FACS patterns of CD8α vs CD11b on gated CD11c+ spleen cells from untreated (UNT) wild-type (n = 6) or Jα18−/− (n = 5) or Batf3−/− (n = 5) or from TLI-conditioned wild-type (n = 11), Batf3−/− (n = 7), or Jα18−/− (n = 8) BALB/c mice, at day 5 after completion of TLI. Percentages of DC subsets in each box are shown. (B) Mean (± standard error of the mean [SEM]) percentages of: CD11c+CD11b−CD8α+ cells (C), CD11c+CD11b+CD8α− cells (D), CD11c+CD11b−CD8α− cells (E). Representative 2-color FACS pattern of 7AAD vs Annexin V staining of spleen cells immediately after completion of 10 doses of TLI in a BALB/c mouse. SSC, side scatter.
Despite these changes in the balance of the DC subsets after TLI, there was no significant change in the mean percentage of total CD11c+MHCIIhi DCs among all spleen cells (data not shown). TLI induced about a 15-fold reduction in the absolute number of nucleated cells7-10 and total DCs in the spleen. There was a considerably larger reduction (about 50-fold) in the absolute number of CD11b−CD8− DCs as compared with the CD11b−CD8+ DC subsets (about 10 fold) that accounted for the altered balance in subsets (supplemental Figure 1A, available on the Blood Web site). The CD8+ DCs were comprised almost entirely of myeloid rather than plasmacytoid DCsbecause only 1% to 2% expressed the PDCA1 marker of plasmacytoid DCs34 (supplemental Figure 1B). The vast majority (86%) of cells remaining in the spleen after 10 doses of TLI were either in the early (Annexin V+ 7-aminoactinomycin D–negative [7AAD−]) or late stages of apoptosis (Annexin V+7AAD+ ) (Figure 2E). Although the percentage of apoptotic cells (Annexin V+) among nucleated spleen cells was similar in untreated mice and in mice given 1 or 2 doses of TLI, there was a gradual increase in apoptotic cells through 10 doses (supplemental Figure 1C).
As expected, the mean percentage of CD11b−CD8+ DCs among total DCs was markedly reduced in the untreated and TLI-treated Batf3−/− spleens (1%-3%). In addition, there was a significant (twofold) reduction in the percentage of CD11b−CD8+ DCs and a concomitant increase in the percentage of CD11b−CD8− DCs among total DCs in untreated wild-type as compared with untreated Jα18−/− mice (Figure 2A,C). This suggests that NKT cells regulate the balance of DC subsets in the normal spleen to favor the CD11b−CD8+ subset. This effect is even more pronounced after TLI treatment because the mean percentage of CD11b−CD8+ DCs did not increase after TLI in NKT-cell–deficient mice Jα18−/− mice (Figure 2A,C) or in Cd1−/− mice (supplemental Figure 2A).
Changes in surface receptor expression on CD8+ DCs after TLI are dependent on NKT cells
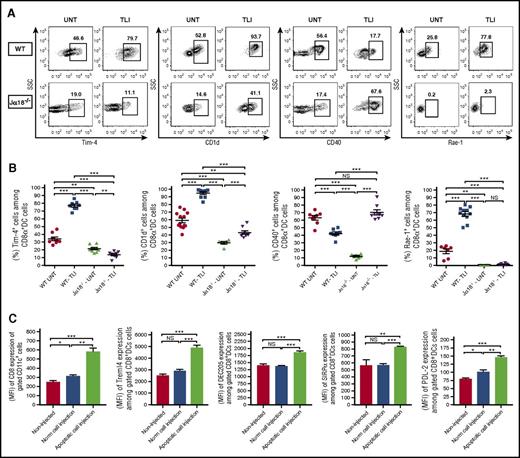
Given the positive impact of NKT cells on CD8+ DC accumulation in TLI-treated mice, we examined the expression of surface receptors on CD8+ DCs including CD1d, Rae-1, and CD40 that interact with the invariant TCRα chain, NKG2D, and CD40L receptors, respectively, on NKT cells.35-37 Figure 3A-B shows that there was a significant increase in the percentage of CD8+ DCs expressing high levels of CD1d and Rae-1 after TLI in wild-type mice, and a significant reduction in the percentage of CD8+ DCs expressing high levels of CD40. These changes were markedly attenuated in NKT-cell–deficient mice with the most dramatic difference seen in the expression of Rae-1 (Figure 3A-B).
Changes in surface receptors of CD8+ DCs after TLI conditioning or injection of apoptotic spleen cells. (A) Shows representative FACS patterns and (B) mean percentages of Tim-4+, CD1d+, CD40+, or Rae-1+ cells on gated splenic CD8+CD11c+ cells from untreated wild-type (n = 8) or Jα18−/− (n = 8) or from TLI conditioned wild-type (n = 8) or Jα18−/− (n = 8) mice at day 5 after completion of conditioning. Bars show mean percentages. (C) Compares the MFI values ± standard error (SE) for staining of CD8, Treml4, DEC205, SIRPα, and PDL-2 receptors on gated CD8+CD11c+ spleen cells from either untreated BALB/c mice (noninjected) (n = 5), BALB/c mice given a single IV injection of whole spleen cells from untreated BALB/c donors (Norm cell injection) (n = 5), or BALB/c mice given a single IV injection of whole spleen cells obtained immediately after 10 daily doses of TLI (apoptotic cell injection) (n = 6). Staining of recipient spleen cells was performed 48 hours after the injection of donor syngeneic spleen cells.
Changes in surface receptors of CD8+ DCs after TLI conditioning or injection of apoptotic spleen cells. (A) Shows representative FACS patterns and (B) mean percentages of Tim-4+, CD1d+, CD40+, or Rae-1+ cells on gated splenic CD8+CD11c+ cells from untreated wild-type (n = 8) or Jα18−/− (n = 8) or from TLI conditioned wild-type (n = 8) or Jα18−/− (n = 8) mice at day 5 after completion of conditioning. Bars show mean percentages. (C) Compares the MFI values ± standard error (SE) for staining of CD8, Treml4, DEC205, SIRPα, and PDL-2 receptors on gated CD8+CD11c+ spleen cells from either untreated BALB/c mice (noninjected) (n = 5), BALB/c mice given a single IV injection of whole spleen cells from untreated BALB/c donors (Norm cell injection) (n = 5), or BALB/c mice given a single IV injection of whole spleen cells obtained immediately after 10 daily doses of TLI (apoptotic cell injection) (n = 6). Staining of recipient spleen cells was performed 48 hours after the injection of donor syngeneic spleen cells.
CD8+ DCs can also interact with apoptotic cells via the Tim-4 receptor that recognizes phosphatidylserine, and via the DEC205 and Treml-4 receptors that recognize other products of apoptotic cell death.38-40 Such interaction with apoptotic cells promotes the induction of tolerogenic DCs.22,41 TLI induced a marked increase in the expression of Tim-4 on CD8+ DCs in wild-type mice (Figure 3A-B) as well as lesser increases in DEC205 and Treml4 (supplemental Figure 3C-D,G-H). The increases were largely lost in NKT-cell–deficient mice (Figure 3A-B; supplemental Figure 3). TLI also increased the expression of the programmed cell death (PD) ligand 2 (PDL-2) ligand for the negative costimulatory PD-1 receptor42 as well as the expression of the phagocytosis-regulating receptor, signal-regulatory protein α (SIRPα),43 on CD8+ DCs in an NKT-cell dependent manner (supplemental Figure 3A-B,E-F).
Splenic DCs failed to express the CD103 marker before and after treatment with TLI (supplemental Figure 3G). As expected,44 the latter marker was expressed on the majority of DCs in the gut (supplemental Figure 3G). PDL-1 expression was not significantly increased after TLI (data not shown). The pattern of changes in DC receptor expression described in the previous 2 paragraphs with TLI alone was similar to that with the combination of TLI and ATS in additional experiments (data not shown).
Injection of naive mice with apoptotic spleen cells from TLI-treated mice induces surface receptor changes in CD8+ DCs like those seen with TLI
Because TLI induced both lymphoid cell apoptosis and changes in DCs, we hypothesized that the recognition and uptake of apoptotic bodies by DCs after TLI contributed to these changes. Accordingly, we harvested the spleens containing 70% to 85% Annexin V+ cells (Figure 2E; supplemental Figure 1C) from wild-type BALB/c mice immediately after 10 doses of TLI, and injected the cells IV into untreated syngeneic recipients. Forty-eight hours later, the intensity of staining of the CD8, DEC205, SIRPα, PDL-2, and Treml4 receptors on recipient DCs had markedly increased as compared with untreated normal controls and recipients of nonirradiated spleen cell injections (Figure 3C). Thus, injection of TLI-induced apoptotic cells changed the pattern of receptor expression on DCs in a fashion similar to that induced by TLI.
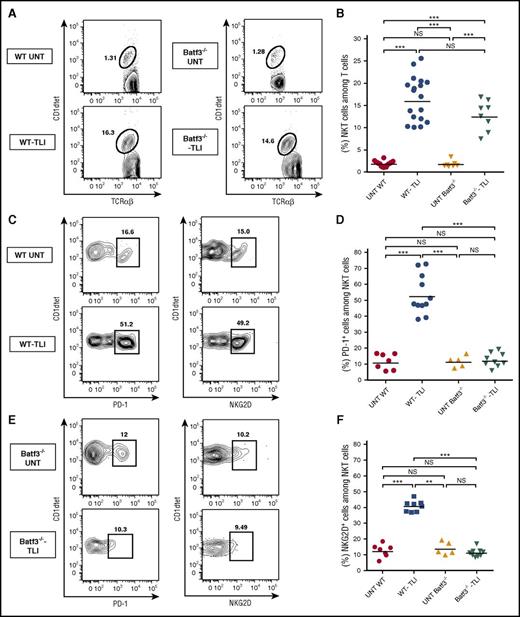
CD8+ DCs regulate the expression of PD-1 and NKG2D on NKT cells
Figure 4A-B shows that the percentage of CD1d tetramer+ NKT cells among total TCRαβ+ T cells increases about eightfold after TLI treatment from a mean of about 2% to 16% in wild-type mice. A similar increase was observed in Batf3−/− mice treated with TLI. In addition to the increase in the percentage of NKT cells, there was a fourfold to fivefold increase in NKT cells that express high levels of surface PD-1 and NKG2D after TLI in wild-type mice (Figure 4C-F). These increases were attenuated in Batf3−/− mice, indicating their dependence on CD8+ DCs. The upregulated Rae-1 receptors on CD8+ DCs can interact with the upregulated NKG2D receptors on NKT cells36 and the upregulated PDL-2 receptors on CD8+ DCs can interact with the upregulated PD-1 receptors on the NKT cells.42 We directly assessed the impact of NKG2D on the phenotypic changes of CD8+ DCs after TLI. Supplemental Figure 4 shows that expression of Rae-1, Treml4, SIRPα, and PDL-2 on CD8+ DCs was significantly increased in wild-type C57BL/6 mice after TLI, but the increase was attenuated in Klrk1−/− C57BL/6 mice.
Increased NKT-cell expression of PD-1 and NKG2D after TLI is attenuated in CD8+ DC–deficient mice. Representative 2-color FACS patterns for CD1dtet (tetramer) vs TCRαβ staining from untreated wild-type (n = 8) or Batf3−/− (n = 5) or TLI-conditioned wild-type (n = 11-16) or Batf3−/− (n = 8-9) mice are shown in panel A. Patterns for CD1dtetramer vs PD-1 or NKG2D staining are shown in panels C and E, respectively. The mean percentages of NKT cells among all T cells, mean percentages of PD-1+ cells among NKT cells, and mean percentages of NKG2D+ cells among NKT cells in the spleen are shown in panels B, D, and F, respectively.
Increased NKT-cell expression of PD-1 and NKG2D after TLI is attenuated in CD8+ DC–deficient mice. Representative 2-color FACS patterns for CD1dtet (tetramer) vs TCRαβ staining from untreated wild-type (n = 8) or Batf3−/− (n = 5) or TLI-conditioned wild-type (n = 11-16) or Batf3−/− (n = 8-9) mice are shown in panel A. Patterns for CD1dtetramer vs PD-1 or NKG2D staining are shown in panels C and E, respectively. The mean percentages of NKT cells among all T cells, mean percentages of PD-1+ cells among NKT cells, and mean percentages of NKG2D+ cells among NKT cells in the spleen are shown in panels B, D, and F, respectively.
CD8+ DCs develop immunosuppressive activity after TLI and induce NKT cells to secrete IL-4
In further experiments, we asked whether TLI causes CD8+ DCs to become immunosuppressive.14-18 In particular, we compared the sorted CD8+ DCs from untreated wild-type BALB/c mice with those obtained after TLI treatment of their ability to suppress the proliferation of normal BALB/c splenic CD4+ and CD8+ T cells in the mixed lymphocyte reaction (MLR) after stimulation with irradiated spleen cells from MHC-mismatched C57BL/6 mice.
Whereas allogeneic stimulator cells alone induced a strong T-cell response (indicated by a marked reduction in CFSE-staining intensity (73% and 67% of CFSElo cells among CD4+ and CD8+ T cells, respectively), the addition of TLI-treated CD8+ DCs to the cultures suppressed proliferation (53% and 44% of CFSElo cells among the CD4+ and CD8+ T cells, respectively). In contrast, the addition of untreated CD8+ DCs had little effect on T-cell proliferation (Figure 5A-B).
CD8+ DCs suppress the MLR, increase IDO production, and retain the capacity to stimulate NKT-cell cytokine secretion. (A) To analyze the in vitro suppression of the proliferation of BALB/c splenic responder CD4+ or CD8+ T cells in the MLR, responder cells were labeled with CFSE (2.5 × 104 per well), and stimulated with irradiated (3000 rad) C57BL/6 splenic cells (5.0 × 104 per well). Sorted CD8+ DCs (2.5 × 104 per well) were or were not added to 5-day cultures. The sorted splenic CD8+ DCs were obtained from untreated (n = 9) or TLI-conditioned wild-type mice (n = 8). Representative plots of CFSE staining with percentages of CFSElo cells are shown. (B) Mean percentages of CD8+ DC suppression of proliferation of CD4+ and CD8+ T cells are shown. (C) Representative 1-color analyses of intensity of staining for IDO among gated CD8+ CD11c+ cells are shown. (D) (MFI) of IDO staining of gated CD8+ DCs from untreated (n = 9) or Jα18−/− (n = 5) or TLI-conditioned wild-type (n = 8) or Jα18−/− (n = 6) mice at day 5 after completion of TLI conditioning is shown. (E) Mean concentrations of IL-4 in supernatants of untreated or TLI-treated splenic CD8+ DCs, and untreated NKT cells that were cultured alone or together for 5 days with or without addition of α-GalCer.
CD8+ DCs suppress the MLR, increase IDO production, and retain the capacity to stimulate NKT-cell cytokine secretion. (A) To analyze the in vitro suppression of the proliferation of BALB/c splenic responder CD4+ or CD8+ T cells in the MLR, responder cells were labeled with CFSE (2.5 × 104 per well), and stimulated with irradiated (3000 rad) C57BL/6 splenic cells (5.0 × 104 per well). Sorted CD8+ DCs (2.5 × 104 per well) were or were not added to 5-day cultures. The sorted splenic CD8+ DCs were obtained from untreated (n = 9) or TLI-conditioned wild-type mice (n = 8). Representative plots of CFSE staining with percentages of CFSElo cells are shown. (B) Mean percentages of CD8+ DC suppression of proliferation of CD4+ and CD8+ T cells are shown. (C) Representative 1-color analyses of intensity of staining for IDO among gated CD8+ CD11c+ cells are shown. (D) (MFI) of IDO staining of gated CD8+ DCs from untreated (n = 9) or Jα18−/− (n = 5) or TLI-conditioned wild-type (n = 8) or Jα18−/− (n = 6) mice at day 5 after completion of TLI conditioning is shown. (E) Mean concentrations of IL-4 in supernatants of untreated or TLI-treated splenic CD8+ DCs, and untreated NKT cells that were cultured alone or together for 5 days with or without addition of α-GalCer.
Previous studies of tolerogenic DCs showed that their immunosuppressive activity can be mediated by the secretion of IDO, a tryptophan-catabolizing enzyme.18 Accordingly, we compared the intracellular expression of IDO in CD8+ DCs in the spleens of untreated and TLI-treated BALB/c mice (Figure 5C). Gated CD8+ DCs from untreated wild-type and untreated Jα18−/− mice exhibited staining intensities that were slightly above the isotype control. The staining intensity increased markedly after TLI treatment of the wild-type mice. There was also an increase after TLI in the Jα18−/− mice that was attenuated as compared with that of wild-type mice. Figure 5D shows that the staining intensity of IDO on gated CD8+ DCs calculated as the mean mean fluorescence intensity (MFI) in wild-type BALB/c mice was significantly increased after TLI compared with that of untreated CD8+ DCs. Interestingly, there was a significant decrease in the IDO staining MFI when comparing the untreated and treated wild-type mice to the untreated and treated Jα18−/− mice (Figure 5D). In order to determine whether the immunosuppressive activity of the TLI-treated wild-type CD8+ DCs was dependent on secretion of IDO, 1M-tryptophan was added to the MLR cultures with the latter DCs. Supplemental Figure 5 shows that addition of tryptophan reversed the suppressive activity of the DCs.
In further experiments, we determined whether in vitro culture of purified CD8+ DCs from untreated or TLI-treated mice will induce untreated NKT cells to increase their secretion of IL-4. Figure 5E shows that IL-4 secretion of CD8+ DCs or NKT cells cultured alone was minimal, and that coculture of either untreated or TLI-treated CD8+ DCs with NKT cells markedly increased IL-4 secretion. The addition of α-GalCer, a potent activator of NKT cells via the invariant TCR,35 augmented IL-4 secretion (Figure 5E).
Secretion of interferon γ (IFNγ) by the NKT cells was significantly lower than that of IL-4 and IL-13 using both untreated or TLI-treated DCs (supplemental Figure 6A-F). However, the ratios of IL-4:IFNγ and IL-13:IFNγ concentrations were significantly increased using TLI-treated as compared with untreated wild-type DCs for stimulation (supplemental Figure 5G). Concentrations of IFNγ in culture supernatants were significantly increased when NKT cells from Klrk1−/− (NKG2D-deficient) were compared with NKT cells from wild-type mice after coculture with wild-type CD8+ DCs (supplemental Figure 6H). The NKG2D deficiency significantly decreased the ratio of IL-4:IFNγ concentrations (supplemental Figure 6I).
Increased expression of PD-1 on both conventional T cells and Tregs after TLI is dependent on CD8+ DCs
As expected from our previous studies,7 there were marked (fourfold to 10-fold) increases in the percentage of PD-1+ cells among splenic gated CD4+ and CD8+ conventional T cells and among gated Tregs in untreated vs TLI-treated wild-type mice in representative stainings (Figure 6A). The percentages of PD-1+ T cells in untreated and TLI-treated Batf3−/− mice were considerably reduced as compared with the wild-type mice in the representative stainings (Figure 6B). The percentages in the TLI-treated Batf3−/− mice were in the range of those in the untreated wild-type mice (Figure 6B).
Kinetics of increased surface marker expression on T cells and CD8+ DCs after TLI in wild-type and Batf3−/−mice. (A) Representative FACS patterns of PD-1 on gated CD4+CD25− conventional T cells (Tcon), or PD-1 on gated CD8+ T cells, or PD-1 on gated CD4+CD25+ Tregs from untreated (n = 8) or TLI-treated wild-type mice (n = 11). (B) Similar stainings are shown in untreated (n = 5) or TLI-treated Batf3−/− mice (n = 10). (C-E) Percentages of PD-1+ cells among T-cell subsets from untreated WT or Batf3−/− mice or from TLI-treated WT or Batf3−/− mice. (F) Percentages of gated splenic CD8+ DCs that expressed Tim-4, DEC205, Treml4, or SIRPα before, during, or after 10 daily doses of TLI conditioning. (G) Percentages of gated NKT, Tregs, conventional CD4+ and conventional CD8+ T cells that expressed PD-1 or NKG2D at the different time points shown in panel F.
Kinetics of increased surface marker expression on T cells and CD8+ DCs after TLI in wild-type and Batf3−/−mice. (A) Representative FACS patterns of PD-1 on gated CD4+CD25− conventional T cells (Tcon), or PD-1 on gated CD8+ T cells, or PD-1 on gated CD4+CD25+ Tregs from untreated (n = 8) or TLI-treated wild-type mice (n = 11). (B) Similar stainings are shown in untreated (n = 5) or TLI-treated Batf3−/− mice (n = 10). (C-E) Percentages of PD-1+ cells among T-cell subsets from untreated WT or Batf3−/− mice or from TLI-treated WT or Batf3−/− mice. (F) Percentages of gated splenic CD8+ DCs that expressed Tim-4, DEC205, Treml4, or SIRPα before, during, or after 10 daily doses of TLI conditioning. (G) Percentages of gated NKT, Tregs, conventional CD4+ and conventional CD8+ T cells that expressed PD-1 or NKG2D at the different time points shown in panel F.
When the mean percentages of PD-1+ cells among CD4+ conventional T cells were compared, there was a statistically significant increase in the TLI-treated vs untreated wild-type mice (Figure 6C). Although there was an increase also in the TLI-treated vs untreated Batf3−/− mice, there was no significant difference in the means of the treated Batf3−/− mice and the untreated wild-type mice (Figure 6C). Similar changes in PD-1 expression were observed in the conventional CD8+ T cells (Figure 6D), and in the Tregs (Figure 6E). Although Treg expression of PD-1 was significantly decreased in the Batf3−/− vs wild-type mice after TLI, the increase in the percentage of Tregs among CD4+ T cells was not significantly different in the 2 groups after TLI (supplemental Figure 4F).
In order to more clearly elucidate the sequence of changes in surface receptors on DCs and T-cell subsets after TLI, we stained these cells before and at serial time points during and after administration of 10 doses of irradiation to determine kinetics of receptor changes. Interestingly, the expression of CD8+ DC receptors that recognize ligands on apoptotic cells (Tim-4, DEC205, Treml4, and SIRP1α) increased rapidly and reached a plateau after the fourth daily dose of TLI (Figure 6F). In contrast, the PD-1 or NKG2D receptor expression on NKT cells, Tregs, and conventional CD4+ and CD8+ T cells rose more slowly, and had not reached a plateau by 5 days after completion of 10 doses of TLI (day 20 after start of treatment) (Figure 6G). It is of interest that the DC surface molecules that interacted with apoptotic bodies increased more rapidly than those that interacted with NKT cells (Rae-1 and PDL-2) (supplemental Figure 4G).
Discussion
The goal of the current study was to further elucidate the molecular and cellular mechanisms by which the TLI and ATG conditioning regimen that is used in both our preclinical and clinical studies promotes chimerism and tolerance.1-11 Prior work with our mouse model has shown that the depletion of conventional T cells following TLI/ATS conditioning is due to p53-dependent apoptotic cell death.10 We hypothesized that host NKT-cell activation and T helper 2 cell (Th2) polarization that is induced by TLI conditioning6,7 is dependent on host CD8+ DCs. Our finding that chimerism and tolerance were abrogated in iNKT-cell–deficient Jα18−/− or Cd1−/− host mice, and in CD8+ myeloid DC–deficient, Batf3−/− mice is consistent with this hypothesis. Because Batf3−/− mice are also deficient in gut, skin, and lung CD103+ DCs, it is possible the latter cells contribute to tolerance despite their absence in the spleen and peripheral lymph nodes.44
Direct evidence for NKT/CD8+ DC interaction was obtained by culturing purified splenic CD8+ DCs from untreated and TLI-treated mice with purified splenic NKT cells in the absence of exogenous glycolipid stimulation. The CD8+ DCs potently induced the NKT cells to secrete IL-4, IL-13, and IFNγ with a Th2 bias that was more profound using the TLI-treated CD8+ DCs. The latter subset became predominant, and “tolerogenic” with increased IDO secretion, and increased ability to suppress the MLR after TLI. IDO has been shown previously to skew NKT-cell cytokine secretion toward IL-4.24
The increased expression of the activation markers PD-1 and NKG2D on NKT cells after TLI treatment of wild-type BALB/c mice was abrogated in the CD8+ DC–deficient (Batf3−/−) mice. In addition, the increased expression of PD-1 on host CD4+CD25+ Tregs, and on conventional CD4+ and CD8+ T cells that has been shown to be dependent on NKT-cell secretion of IL-4 after TLI,6,7 was markedly attenuated in the CD8+ DC–deficient mice. Thus, the NKT-cell activation and the downstream tolerogenic effects of activation were dependent upon CD8+ DCs.
Changes in DC receptor expression after TLI were markedly reduced in NKT-cell–deficient (Jα18−/−) mice, indicating that there is a mutual regulation of CD8+ DC and NKT-cell activation. In addition, deficiency of NKG2D receptors abrogated chimerism and tolerance, attenuated the changes in DC receptor expression, and attenuated the Th2 bias of NKT cells after interaction in vitro with the CD8+ DCs. The latter findings suggest that the tolerogenic CD8+ DC/NKT-cell interactions are dependent on the NKG2D receptors on NKT cells and their ligands on the DCs.
We asked whether the injection of TLI-generated apoptotic spleen cells into untreated mice could mimic the DC receptor upregulation observed after TLI. Following the injection, there was a significant increase in the expression of DEC205, Treml4, PDL-2, and SIRPα receptors on CD8+ DCs as was the case following TLI. It is of interest that injection of recipient or donor strain apoptotic cells, generated ex vivo, into sublethally total-body irradiated recipients of bone marrow transplants facilitated chimerism and tolerance.45
The results of the current study, combined with prior data, confirm that interactions with multiple innate and adaptive immune cell types are required for the induction of chimerism and tolerance following TLI/ATG.6-9 Figure 7 identifies these interactions and their postulated chronology based on observation of the kinetics of surface molecule changes reported herein. CD8+ DCs are likely responsible for the initiation of tolerance induction because these cells were the most rapidly activated upon uptake of apoptotic cells, and TLI induces massive lymphocyte apoptosis and DNA damage.6,7,10 The activated DCs become functionally immunosuppressive, due in part to their production of IDO, and interact with and activate the host NKT cells to polarize these cells toward IL-4 secretion.6,7 The activated NKT cells in turn promote tolerance by interacting with a variety of residual host innate and adaptive immune cells including myeloid-derived suppressor cells (MDSCs), Tregs, and conventional CD4+ and CD8+ T cells in an IL-4–dependent manner.6,7 These interactions increase the immune-suppressive functions of the latter cells by augmenting their secretion of inhibitory molecules such as arginase-1, and IL-10, and by upregulating negative costimulatory molecules such as PD-1 on all T-cell subsets.7,30 The ability of the residual host conventional T cells to reject the infused donor hematopoietic cells is suppressed, thereby, promoting chimerism and central clonal deletion.8,9
Summary of cell interactions and molecular products that prevent rejection of combined donor hematopoietic cell and organ transplants after conditioning with TLI and ATS. Lymphoid tissue radiation induces massive cell death and generation of apoptotic bodies that are recognized by host CD8+ DC receptors including Tim-4, DEC205, SIRPα, and Treml4. After TLI, the DCs become “tolerogenic” with IDO production, and upregulate surface molecules CD1d, Rae-1, and PDL-2 that interact with TCR, NKG2D, and PD-1 receptors on host NKT cells. NKT cells polarize their cytokine production toward IL-4, and interact with host Tregs to upregulate PD-1 and polarize their cytokine production toward IL-10. The “tolerogenic” NKT cells also activate host MDSCs to upregulate PDL-1 and IL-4Rα, and to secrete arginase-1. Activated innate and adaptive host immune cells suppress rejection by host Tcons, and promote chimerism and organ graft acceptance. HCT, hematopoietic cell transplantation.
Summary of cell interactions and molecular products that prevent rejection of combined donor hematopoietic cell and organ transplants after conditioning with TLI and ATS. Lymphoid tissue radiation induces massive cell death and generation of apoptotic bodies that are recognized by host CD8+ DC receptors including Tim-4, DEC205, SIRPα, and Treml4. After TLI, the DCs become “tolerogenic” with IDO production, and upregulate surface molecules CD1d, Rae-1, and PDL-2 that interact with TCR, NKG2D, and PD-1 receptors on host NKT cells. NKT cells polarize their cytokine production toward IL-4, and interact with host Tregs to upregulate PD-1 and polarize their cytokine production toward IL-10. The “tolerogenic” NKT cells also activate host MDSCs to upregulate PDL-1 and IL-4Rα, and to secrete arginase-1. Activated innate and adaptive host immune cells suppress rejection by host Tcons, and promote chimerism and organ graft acceptance. HCT, hematopoietic cell transplantation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Kent Jensen and Bianca Gomez for their assistance with data analysis using the Luminex machine and FACS sort, respectively. In addition, the authors also thank P. Savage (Brigham Young University, Provo, UT) for providing us with the α-GalCer, and the NIH Tetramer Facility (Rockville, MD) for providing the CD1d-tetramer.
This work was supported by National Institutes of Health National Institute of Allergy and Infectious Diseases research grant RO1-AI-037683, National Cancer Institute research grant RO1-CA-16344105, and National Heart, Lung, and Blood Institute research grant PO1-HL-075462.
Authorship
Contribution: D.H. designed and performed research, contributed vital analytical methods, collected, analyzed and interpreted data, and wrote the manuscript; X.T. purified NKT and DCs, and helped perform in vitro dendritic /NKT-cell and apoptosis experiments; X.Z. helped perform in vitro DC cytokine experiments; E.G.E. helped design experiments and wrote the manuscript; and S.S. provided overall research supervision and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Samuel Strober, Division of Immunology & Rheumatology/Medicine, Stanford University School of Medicine, CCSR Building, Room 2215-C, 269 Campus Dr, Stanford, CA 94305-5166; e-mail: sstrober@stanford.edu.

![Figure 1. CD8+ DCs, NKT cells, and expression of NKG2D are required for heart allograft survival and development of chimerism after combined BMT and heart transplantation. (A) Experimental scheme: BALB/c or C57BL/6 hosts were given donor C57BL/6 or BALB/c neonatal heart transplants (HTX), respectively, on day 0, and ATS was injected i.p. on days 0, 2, 6, 8, and 10. Hosts were conditioned over 14 days with 10 doses of TLI of 240 cGy each. On day 15, 50 × 106 C57BL/6 or BALB/c donor bone marrow cells from the same strain as the heart grafts were injected IV (bone marrow transplantation [BMT]), and chimerism and heart graft survival were monitored for 100 days after organ transplantation. (B) Percentages of hosts with heart graft survival among wild-type (WT) BALB/c (n = 10), or Batf3−/− (n = 8), or Jα18−/− (n = 8) hosts or WT C57BL/6 (n = 10) or NKG2D-deficient (Klrk1−/−) (n = 8) hosts at serial time points. WT TX (BALB/c) vs Batf3−/− TX: P < .001; WT TX (BALB/c) vs Jα18−/− TX: P < .001; WT TX (B6) vs NKG2D-deficient (Klrk1−/−) TX (B6): P < .001; Batf3−/− TX vs Jα18−/− TX: P < .001 (log-rank; Mantel-Cox test). (C) Percentages of donor type cells among (T cells, B cells, macrophages, and granulocytes in the blood of hosts 28 days after BMT. Bars show mean percentages of donor cells. P values by the 2-tailed t test of independent means. *P < .05; **P < .01; ***P < .001; NS, not significant (P > .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/12/10.1182_blood-2016-07-723015/4/m_blood723015f1.jpeg?Expires=1767859179&Signature=P~f7Z3bDHJKNG~TAizo70CJthfAPTzmr1A2pbh8yXLsa5JMeZ-U6QAeTVfMMN-SNYOziBD37jxwIvn5lAloSrmOhHDflmwLMuK8oD5OZzsKMkjwzd5ewenMr6RdbD4ThTRGF4HNDaJ-kTxHiIzVwMVe2aibrozON4b8Z-PsgNeoM0EhTZJBGo~zpKvXMovo7m9~4SGW17cuvwTBm3-SbMtWg8z7s34GQSZCydiRAgbZW~3uKdlKnupsazFhA4KW~~4O5lVoAP2L2htArlmlt-srkAXys~q6Fsi6g2nJ~a-ZvGz2QI~Aj6QD1gAUmy~B5~QKoeK4It6zD338vei7dgg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. TLI conditioning that favors the accumulation of CD8+ DCs is dependent on NKT cells. (A) Representative 2-color FACS patterns of CD8α vs CD11b on gated CD11c+ spleen cells from untreated (UNT) wild-type (n = 6) or Jα18−/− (n = 5) or Batf3−/− (n = 5) or from TLI-conditioned wild-type (n = 11), Batf3−/− (n = 7), or Jα18−/− (n = 8) BALB/c mice, at day 5 after completion of TLI. Percentages of DC subsets in each box are shown. (B) Mean (± standard error of the mean [SEM]) percentages of: CD11c+CD11b−CD8α+ cells (C), CD11c+CD11b+CD8α− cells (D), CD11c+CD11b−CD8α− cells (E). Representative 2-color FACS pattern of 7AAD vs Annexin V staining of spleen cells immediately after completion of 10 doses of TLI in a BALB/c mouse. SSC, side scatter.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/12/10.1182_blood-2016-07-723015/4/m_blood723015f2.jpeg?Expires=1767859179&Signature=xuOBlB2nImLG2KsDkB7dmtZZgRpFcvxK9d2ftMBlsnPcBv6bOFHXjgcVKUZQ2x5vtmF7Vjm1kHH2z4kUNyRte~I4HxPzIP5PI5FNJ3B29DMaeZd9npOeYwm3gBRF5Xr4BnThAwI3Rbmu~L2q8zKoe~06IHpOlyxWL1jK-PvYNIzyEc7Q5Z0zzz7V3R4FsUzjOVTSeyok~mkK3vndxfhNPn3vD8IHyAPTplCxuJAsvTkhjmZgXhEntJ3s7YurDx1XaammVZapYF6gG9CXs9Lno86dpaQZlSTbfThNiD9gC19Jd~u4K9CFpn7-09n6PJdfOD-2LL1NexJrh02IjVjqXg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)