Key Points
The plasma contact system is activated early in AD mice and temporally correlated with the onset of brain inflammation.
Depletion of contact system initiator FXII ameliorates brain pathology and cognitive impairment in AD mice.
Abstract
Vascular abnormalities and inflammation are found in many Alzheimer disease (AD) patients, but whether these changes play a causative role in AD is not clear. The factor XII (FXII) –initiated contact system can trigger both vascular pathology and inflammation and is activated in AD patients and AD mice. We have investigated the role of the contact system in AD pathogenesis. Cleavage of high-molecular-weight kininogen (HK), a marker for activation of the inflammatory arm of the contact system, is increased in a mouse model of AD, and this cleavage is temporally correlated with the onset of brain inflammation. Depletion of FXII in AD mice inhibited HK cleavage in plasma and reduced neuroinflammation, fibrinogen deposition, and neurodegeneration in the brain. Moreover, FXII-depleted AD mice showed better cognitive function than untreated AD mice. These results indicate that FXII-mediated contact system activation contributes to AD pathogenesis, and therefore this system may offer novel targets for AD treatment.
Introduction
Alzheimer disease (AD) is a fatal cognitive disorder that results in neuronal degeneration. Despite extensive investigation, a huge gap exists in our understanding of the pathology of AD. Amyloid beta (Aβ) is generally recognized as a primary driver of the disease.1,2 It is also known that AD patients suffer extensive neuronal death. The gap is the link between Aβ and neuronal degeneration.
It is therefore important for our understanding of AD to consider pathways that can lead to neuronal death and determine whether Aβ can influence these pathways. Most AD patients suffer from vascular abnormalities3-12 and neuroinflammation,13-15 and there is strong evidence that these pathologies are early core pathologies of AD. Both vascular abnormalities and inflammation can trigger neuronal death, but it is not clear whether Aβ can affect these pathologies.
The contact system, driven by factor XII (FXII), can launch both prothrombotic (through activation of factor XI [FXI]) and proinflammatory (through activation of plasma prekallikrein [PPK]) pathways, leading to the release of bradykinin from high-molecular-weight kininogen (HK).16 This system could play a role in the vascular and inflammatory aspects of AD pathology. We and others have shown that Aβ can initiate the contact system in vitro and in vivo,17-20 and AD patients and AD mice both have evidence of a contact activation.19,21
In this study, we show that increased HK cleavage, a marker for activation of the inflammatory arm of the contact pathway, is temporally correlated in AD mice with the onset of brain inflammation. Depletion of plasma FXII, the initiator of the contact pathway, using antisense oligonucleotide (ASO)–mediated messenger RNA knockdown, inhibited HK cleavage in AD mouse plasma and reduced neuroinflammation, fibrin(ogen) deposition, and neuronal degeneration in the brain. Moreover, FXII-ASO–treated AD mice showed better cognitive function than control ASO (CTL-ASO) –treated AD mice. Therefore, our results provide a mechanistic link between Aβ and neuroinflammation and vascular abnormities, which could result in neuronal degeneration.
Methods
Animals
All animal experiments were conducted in accordance with the guidelines of the US National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and with approval from the Animal Care and Use Committee of The Rockefeller University. TgCRND8 transgenic mice (provided by A. Chishti and D. Westaway, University of Toronto, Toronto, ON, Canada) have three APP mutations (K670N, M671L, and V717F) driven by the human prion protein promoter.22 In all experiments, wild-type (WT) littermates were used as controls. For plasma HK and brain inflammation analyses, WT and AD mice at 2, 3, and 6 months of age were used (n = 12 per group per age). FXII-ASO (murine sequence)23 and CTL-ASO (no homologies to the mouse genome) were dissolved in saline and injected subcutaneously at a dose of 150 mg/kg per week for the first 2 weeks and then 50 mg/kg per week until the end of the study. Two-month-old WT and AD mice were treated with FXII-ASO or CTL-ASO for 4 months (n = 9-14 mice per group). Plasma was collected the day before the treatment began (by tail clipping) and the day the mice were euthanized (retro-orbitally). In a pilot experiment, plasma was collected at 2 weeks, 2 and 3 months after treatment, and at the end of the treatment for confirmation of FXII depletion by western blots. Only male mice were used in the experiments.
Behavioral analysis
All behavioral experiments were performed and analyzed by a researcher blinded to genotype and treatment. Mice were acclimated to the testing room for 6 days before the experiments and handled for 15 minutes per day for 6 days before the test.
Barnes maze and contextual fear conditioning
Plasma preparation
Blood was collected through either the retro-orbital plexus or by tail clipping. For retro-orbital bleeding, capillary tubes were coated with Gel Repel (Z719951; Sigma) and 2.5 mg/mL polybrene (SC-134220; Santa Cruz) to block the negatively charged surface. Mice were anesthetized, their retro-orbital plexus was penetrated with the coated capillary tube, and blood was collected into EDTA-coated tubes (BD Microtainer). Plasma was prepared by centrifugation and frozen on dry ice. For tail clipping, a very small piece of soft tissue at the end of the tail was clipped. Blood (20-30 μL) was collected into EDTA-coated tubes for plasma preparation.
Immunohistochemistry
Immunohistochemistry was performed as described.26 In brief, mice were deeply anesthetized and perfused with saline, and their brains were collected. Brain hemispheres were either prepared for western blots or fixed in 2% paraformaldehyde for brain sectioning and immunohistochemical analysis. Primary antibodies used were against fibrinogen (Dako), CD11b (Developmental Studies Hybridoma Bank), ionized calcium-binding adapter molecule 1 (Iba-1, Wako), glial fibrillary acidic protein (GFAP, Dako), neuron-specific class III β-tubulin (Tuj1, Covance), NeuN (Millipore), and Aβ (BioLegend). Brain sections were incubated with primary antibodies at 4°C overnight, rinsed in phosphate-buffered saline, and then incubated with the appropriate fluorescent dye–conjugated second antibody.
Western blot analysis
Western blots were performed as described.27 Brain tissues were homogenized on ice in 2% sodium dodecyl sulfate, 95 mM NaCl, 25 mM tris(hydroxymethyl)aminomethane (pH 7.4), 10 mM EDTA, and protease inhibitor cocktail (Roche). After centrifugation, extracts were collected for western blot. Samples were run on reducing sodium dodecyl sulfate polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membrane (EMD Millipore), incubated overnight at 4°C in primary antibody (rat anti-HK light chain, R&D Systems; goat anti-FXII, Cedarlane; goat anti-PPK, R&D Systems; rabbit anti-transferrin, Abcam; rabbit anti-GFAP, DAKO; and rabbit anti-Iba-1, Wako), and then incubated with horseradish peroxidase–conjugated secondary antibody. Blots were developed with Enhanced Chemoluminescent Substrate (Perkin-Elmer). After stripping with Restore Plus Western Blot Stripping Buffer (Thermo Scientific) at room temperature for 15 minutes, the membranes were reblotted with transferrin antibody (Abcam) for plasma samples or glyceraldehyde-3-phosphate dehydrogenase antibody (Abcam) for brain protein extracts. Protein levels were quantified by using densitometry with NIH Image J. Western blot results for plasma samples were normalized to transferrin, and brain protein extracts were normalized to glyceraldehyde-3-phosphate dehydrogenase.
Imaging analysis
After immunostaining, brain sections were examined with a microscope (Axiovert 200; Carl Zeiss) equipped with Plan-Neofluar (10× NA 0.3, 20× NA 0.5, and 40× NA 0.75) objective lenses at room temperature. The imaging medium was air for all the objective lenses used. The AxioCam color camera (Carl Zeiss) and AxioVision software (Carl Zeiss) were used for image collection. Each set of stained sections was processed for images under identical settings. Figures were prepared by using Adobe Photoshop and PowerPoint. For quantification of fluorescence staining intensity, images were acquired and thresholded by using NIH Image J. The total area of positive staining was analyzed as a percentage of total cortex area, and the analyzer was blinded to the genotype and treatment of mice. An average of 3 to 4 different sections from each mouse were analyzed (n = 9-14 mice per group)
Statistical analysis
GraphPad Prism software was used for all statistical analyses with Student two-tailed t test, one-way analysis of variance, or two-way analysis of variance. All numerical values presented in graphs are mean ± standard error of the mean.
Results
The plasma contact system is activated at early stages in AD mice and temporally correlated with astrocyte and microglia/macrophage activation in the brain
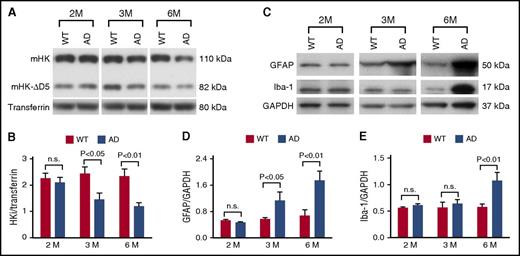
To analyze the time course of plasma contact system activation in TgCRND8 AD mice (hereafter designated AD mice),22 we collected plasma at 2, 3, and 6 months of age (n = 12 mice per group per age) and examined intact HK (HKi, which is the sum of mouse HK [mHK] and mouse HK without domain 5 [mHK-ΔD5]) levels by western blot. At 2 months of age, HKi levels were similar between AD mice and their WT littermates (Figure 1A-B). However, by 3 months of age, HKi levels were significantly lower in AD mice compared with their WT littermates (Figure 1A-B). At 6 months of age, HKi levels in AD mice were reduced even further (Figure 1A-B). We also analyzed changes of intact mHK and mHK-ΔD5 separately, and both showed a similar significant decrease in AD mouse plasma at 3 and 6 months of age, but not at 2 months of age (supplemental Figure 1, available on the Blood Web site). These results indicate HK consumption, which is an indication of contact system activation at early stages of disease progression in AD mice.
The plasma contact system is activated at early disease stages in AD mice and temporally correlated with astrocyte and microglia/macrophage activation in the brain. (A) Representative western blots probed with an antibody against mouse HK (mHK) light chain showing plasma HK levels in AD mice and their WT littermates at 2, 3, and 6 months of age. (B) The levels of HKi (sum of mHK and mHK-∆D5 bands) normalized to transferrin were similar in AD mice compared with WT littermates at 2 months of age, but levels of HKi were significantly lower in AD mice compared with WT littermates at 3 and 6 months of age. (C) Representative western blots probed with antibodies against GFAP and Iba-1 show astrocyte and microglia/macrophage activation in AD and WT mice at 2, 3, and 6 months of age. (D) GFAP expression was similar between AD mice and their WT littermates at 2 months of age, but was significantly increased in AD mouse brain compared with that of WT littermates at 3 and 6 months of age, indicating the onset and prolonged activation of astrocytes in the AD mouse brain. (E) At 2 and 3 months of age, expression levels of Iba-1 were similar between AD mice and their WT littermates. However, microglia/macrophage activation was significantly higher in AD mouse brain compared with brains of WT mice at 6 months of age. Student t test; n = 12 mice per group per age. All values presented as mean ± standard error of the mean (SEM). Results are from 2 independent experiments. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; n.s., not significant.
The plasma contact system is activated at early disease stages in AD mice and temporally correlated with astrocyte and microglia/macrophage activation in the brain. (A) Representative western blots probed with an antibody against mouse HK (mHK) light chain showing plasma HK levels in AD mice and their WT littermates at 2, 3, and 6 months of age. (B) The levels of HKi (sum of mHK and mHK-∆D5 bands) normalized to transferrin were similar in AD mice compared with WT littermates at 2 months of age, but levels of HKi were significantly lower in AD mice compared with WT littermates at 3 and 6 months of age. (C) Representative western blots probed with antibodies against GFAP and Iba-1 show astrocyte and microglia/macrophage activation in AD and WT mice at 2, 3, and 6 months of age. (D) GFAP expression was similar between AD mice and their WT littermates at 2 months of age, but was significantly increased in AD mouse brain compared with that of WT littermates at 3 and 6 months of age, indicating the onset and prolonged activation of astrocytes in the AD mouse brain. (E) At 2 and 3 months of age, expression levels of Iba-1 were similar between AD mice and their WT littermates. However, microglia/macrophage activation was significantly higher in AD mouse brain compared with brains of WT mice at 6 months of age. Student t test; n = 12 mice per group per age. All values presented as mean ± standard error of the mean (SEM). Results are from 2 independent experiments. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; n.s., not significant.
To investigate whether there is a relationship between plasma contact system activation and brain inflammation, we analyzed activation of astrocytes (using GFAP) and microglia/macrophages (using Iba-1 and CD11b) in the mouse brain. Both Iba-1 and CD11b are markers for microglia and monocytes/macrophages, so the presence of these antigens could indicate activation of brain microglia or infiltration of circulating monocytes/macrophages into the brain; hereafter, we refer to Iba-1– and/or CD11b-positive cells as microglia/macrophages. At 2 months of age, there was no significant difference in expression levels of GFAP and Iba-1 between AD and WT littermates (Figure 1C-E) by western blot. However, by 3 months of age, GFAP expression level was significantly increased in AD mouse brains compared with WT mouse brains, whereas Iba-1 expression level did not increase significantly (Figure 1C-E). At 6 months of age, expression levels of both GFAP and Iba-1 were significantly increased in AD compared with WT mouse brain (Figure 1C-E). These results demonstrate a temporal correlation between the activation of the plasma contact system and the onset of brain parenchymal astrocyte and microglia/macrophage activation, suggesting that contact system activation could contribute to neuroinflammation in AD.
FXII-mediated contact system activation in AD mice
Our results showed that the levels of HKi in AD mice were decreased at the early stages of the disease. To investigate whether decreased HKi in AD mice was mediated by FXII activation, we used an ASO-mediated gene knockdown strategy to deplete FXII in AD and WT mice. ASO directed against FXII can efficiently and specifically deplete mouse FXII.23 Treatment with FXII-ASO or CTL-ASO was initiated at 2 months of age and continued for 4 months (n = 9-14 mice per group).
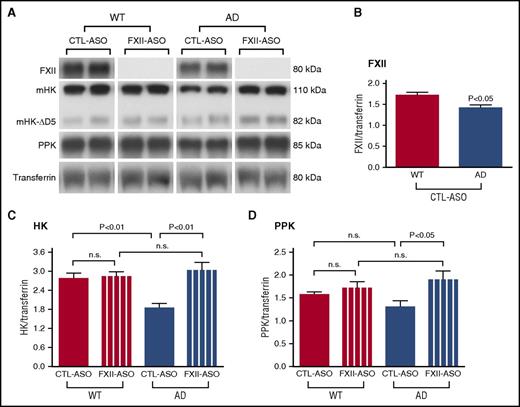
Plasma FXII was not detectable by western blot in FXII-ASO–treated AD or WT mice (Figure 2A), indicating that ASO treatment efficiently depleted the targeted protein. Plasma FXII in CTL-ASO–treated AD mice was significantly lower when compared with CTL-ASO–treated WT mice (Figure 2B), suggesting that FXII may undergo activated-dependent consumption in the AD mouse plasma.
FXII-mediated contact system activation in AD mouse plasma. AD mice and their WT littermate controls were treated with FXII-ASO or CTL-ASO for 4 months. Blood was collected at the end of the treatment, and FXII, PPK, and HK levels were determined by western blot. Transferrin was used to normalize the samples. (A) Representative western blots (2 samples per group). (B) Plasma FXII level in CTL-ASO-treated AD mice was significantly lower when compared to that of CTL-ASO–treated WT mice (Student t test, n = 10 mice per group). Values are presented as mean ± SEM. Results are from 3 independent experiments. (C) HKi levels in WT mouse plasma were similar between groups, and HKi levels in CTL-ASO–treated AD mice were significantly lower than those in CTL-ASO–treated WT mice. In FXII-ASO–treated AD mice, HKi levels were significantly higher compared to CTL-ASO–treated AD mice, but similar to FXII-ASO–treated WT mice. (D) PPK levels were similar in WT mice between groups, but PPK levels in FXII-ASO–treated AD mice were significantly higher than those in CTL-ASO–treated AD mice. For (C) and (D), one-way analysis of variance, (ANOVA), n = 9-14 mice per group; all values presented as mean ± SEM. Results are from 3 independent experiments.
FXII-mediated contact system activation in AD mouse plasma. AD mice and their WT littermate controls were treated with FXII-ASO or CTL-ASO for 4 months. Blood was collected at the end of the treatment, and FXII, PPK, and HK levels were determined by western blot. Transferrin was used to normalize the samples. (A) Representative western blots (2 samples per group). (B) Plasma FXII level in CTL-ASO-treated AD mice was significantly lower when compared to that of CTL-ASO–treated WT mice (Student t test, n = 10 mice per group). Values are presented as mean ± SEM. Results are from 3 independent experiments. (C) HKi levels in WT mouse plasma were similar between groups, and HKi levels in CTL-ASO–treated AD mice were significantly lower than those in CTL-ASO–treated WT mice. In FXII-ASO–treated AD mice, HKi levels were significantly higher compared to CTL-ASO–treated AD mice, but similar to FXII-ASO–treated WT mice. (D) PPK levels were similar in WT mice between groups, but PPK levels in FXII-ASO–treated AD mice were significantly higher than those in CTL-ASO–treated AD mice. For (C) and (D), one-way analysis of variance, (ANOVA), n = 9-14 mice per group; all values presented as mean ± SEM. Results are from 3 independent experiments.
We then analyzed HKi changes between WT and AD mice treated with FXII-ASO or CTL-ASO (Figure 2A,C). In WT mouse plasma, HKi levels were similar between groups, indicating that FXII does not mediate significant HK cleavage in the normal WT system. This result is consistent with FXII−/− mice, which have HKi levels similar to those of WT mice.28 HKi levels in CTL-ASO–treated AD mice, as expected, were significantly lower than those in CTL-ASO–treated WT mice. Importantly, in FXII-ASO–treated AD mice, HKi levels were higher compared with CTL-ASO–treated AD mice, but similar to FXII-ASO–treated WT mice, demonstrating that FXII was activated and that it mediated significant HK cleavage in AD mice.
We also analyzed PPK changes in AD and WT mice treated with FXII-ASO or CTL-ASO (Figure 2A,D). In WT mice, PPK levels were similar in mice treated with CTL-ASO or FXII-ASO, indicating that FXII did not mediate significant PPK activation in WT mice. In CTL-ASO–treated AD mice, PPK levels were lower, suggesting consumption of the protein. However, treatment of AD mice with FXII-ASO restored the PPK levels to that observed in WT mice, indicating that the lower levels of PPK in AD mice were the result of FXII activity.
Depletion of plasma FXII reduced brain inflammation in AD mice
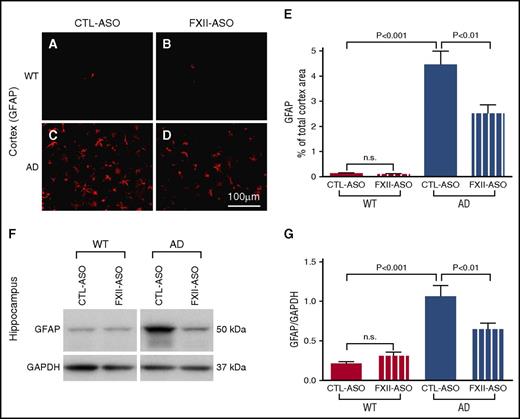
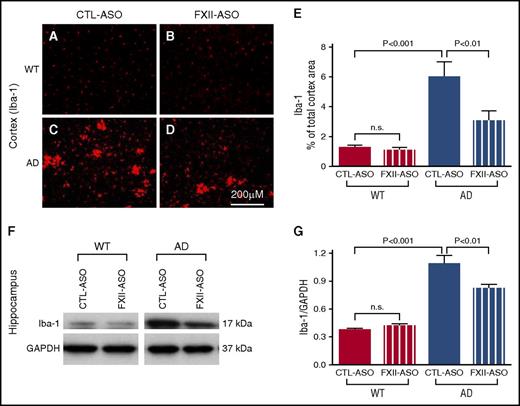
Because we observed a correlation between activation of the plasma contact system and the onset of brain parenchymal inflammation in AD mice, we tested whether activation of the plasma contact system contributes to brain inflammation. If so, then depletion of FXII in plasma, which reduced activation of the contact system and HK cleavage in AD mice, might reduce brain inflammation. To investigate this possibility, we compared GFAP and Iba-1 expression levels among WT and AD mice treated with CTL-ASO or FXII-ASO in the cerebral cortex and hippocampus (n = 9-14 mice per group) (Figures 3 and 4). The expression levels of GFAP and Iba-1 were similar between WT mice treated with CTL-ASO or FXII-ASO. In CTL-ASO–treated AD mice, both GFAP and Iba-1 levels were significantly higher than in WT mice, indicating neuroinflammation in AD mice. However, AD mice treated with FXII-ASO showed significantly lower levels of GFAP and Iba-1 expression than AD mice treated with CTL-ASO, signifying that depletion of FXII reduced neuroinflammation in the AD mice. These results show that depletion of FXII in the plasma reduced AD-related inflammation in the brain, suggesting that activation of the plasma contact system contributes to neuroinflammation in AD.
Depletion of plasma FXII reduces astrocyte activation in the AD mouse brain. (A-E) Brain sections from WT and AD mice treated with CTL-ASO or FXII-ASO were stained with an antibody against GFAP (A-D), and the cerebral cortex was analyzed (E). CTL-ASO–treated AD mice showed significantly higher expression of GFAP (C,E) than CTL-ASO–treated WT mice (A,E). In FXII-ASO–treated AD mice, GFAP (D) was significantly reduced compared with CTL-ASO–treated AD mice (C,E). GFAP expression was similar between CTL-ASO–treated (A,E) and FXII-ASO–treated (B,E) WT mice (one-way ANOVA; n = 9-14 mice per group). Scale bar for panels A-D, 100 μm. (F-G) Western blot analyses of hippocampal extracts from WT and AD mice treated with CTL-ASO or FXII-ASO revealed that the expression level of GFAP was significantly higher in CTL-ASO–treated AD mice than in CTL-ASO–treated WT mice. FXII-ASO treatment significantly reduced GFAP expression in AD mice when compared with CTL-ASO treatment. The expression level of GFAP was similar between WT mice treated with FXII-ASO or CTL-ASO (one-way ANOVA; n = 9-14 mice per group; shown here are representative western blots). All values presented as mean ± SEM. Results are from 3 independent experiments.
Depletion of plasma FXII reduces astrocyte activation in the AD mouse brain. (A-E) Brain sections from WT and AD mice treated with CTL-ASO or FXII-ASO were stained with an antibody against GFAP (A-D), and the cerebral cortex was analyzed (E). CTL-ASO–treated AD mice showed significantly higher expression of GFAP (C,E) than CTL-ASO–treated WT mice (A,E). In FXII-ASO–treated AD mice, GFAP (D) was significantly reduced compared with CTL-ASO–treated AD mice (C,E). GFAP expression was similar between CTL-ASO–treated (A,E) and FXII-ASO–treated (B,E) WT mice (one-way ANOVA; n = 9-14 mice per group). Scale bar for panels A-D, 100 μm. (F-G) Western blot analyses of hippocampal extracts from WT and AD mice treated with CTL-ASO or FXII-ASO revealed that the expression level of GFAP was significantly higher in CTL-ASO–treated AD mice than in CTL-ASO–treated WT mice. FXII-ASO treatment significantly reduced GFAP expression in AD mice when compared with CTL-ASO treatment. The expression level of GFAP was similar between WT mice treated with FXII-ASO or CTL-ASO (one-way ANOVA; n = 9-14 mice per group; shown here are representative western blots). All values presented as mean ± SEM. Results are from 3 independent experiments.
Microglia/macrophage activation is reduced in AD mice treated with FXII-ASO. (A-E) Brain sections from WT and AD mice treated with CTL-ASO or FXII-ASO were stained with antibodies against Iba-1, and the cerebral cortex was analyzed (A-E). CTL-ASO–treated AD mice showed significantly higher expression of Iba-1 (C,E) than CTL-ASO–treated WT mice (A,E). In FXII-ASO–treated AD mice, Iba-1expression (D-E) was significantly reduced compared with CTL-ASO–treated AD mice (C,E). Iba-1 expression was similar between CTL-ASO–treated (A,E) and FXII-ASO–treated WT mice (B,E) (one-way ANOVA; n = 9-14 mice per group). Scale bar for panels A-D, 200 μm. (F-G) Western blot analyses of hippocampal extracts from WT and AD mice treated with CTL-ASO or FXII-ASO showed that the expression level of Iba-1 was significantly higher in CTL-ASO–treated AD mice than in CTL-ASO–treated WT mice. FXII-ASO treatment significantly reduced Iba-1 expression in AD mice when compared with CTL-ASO treatment. The expression level of Iba-1 was similar between WT mice treated with FXII-ASO or CTL-ASO (one-way ANOVA; n = 9-14 mice per group; shown here are representative western blots). All values presented as mean ± SEM. Results are from 3 independent experiments.
Microglia/macrophage activation is reduced in AD mice treated with FXII-ASO. (A-E) Brain sections from WT and AD mice treated with CTL-ASO or FXII-ASO were stained with antibodies against Iba-1, and the cerebral cortex was analyzed (A-E). CTL-ASO–treated AD mice showed significantly higher expression of Iba-1 (C,E) than CTL-ASO–treated WT mice (A,E). In FXII-ASO–treated AD mice, Iba-1expression (D-E) was significantly reduced compared with CTL-ASO–treated AD mice (C,E). Iba-1 expression was similar between CTL-ASO–treated (A,E) and FXII-ASO–treated WT mice (B,E) (one-way ANOVA; n = 9-14 mice per group). Scale bar for panels A-D, 200 μm. (F-G) Western blot analyses of hippocampal extracts from WT and AD mice treated with CTL-ASO or FXII-ASO showed that the expression level of Iba-1 was significantly higher in CTL-ASO–treated AD mice than in CTL-ASO–treated WT mice. FXII-ASO treatment significantly reduced Iba-1 expression in AD mice when compared with CTL-ASO treatment. The expression level of Iba-1 was similar between WT mice treated with FXII-ASO or CTL-ASO (one-way ANOVA; n = 9-14 mice per group; shown here are representative western blots). All values presented as mean ± SEM. Results are from 3 independent experiments.
We also compared Aβ deposition between AD mice treated with CTL-ASO or FXII-ASO, and there was no significant difference (supplemental Figure 2). However, the immunohistochemical staining for CD11b and GFAP proximal to Aβ plaques was significantly reduced in FXII-ASO–treated AD mice, indicating that treatment with FXII-ASO mainly affects glial cell activation (supplemental Figure 2).
FXII-ASO reduced fibrin deposition in AD mouse brains
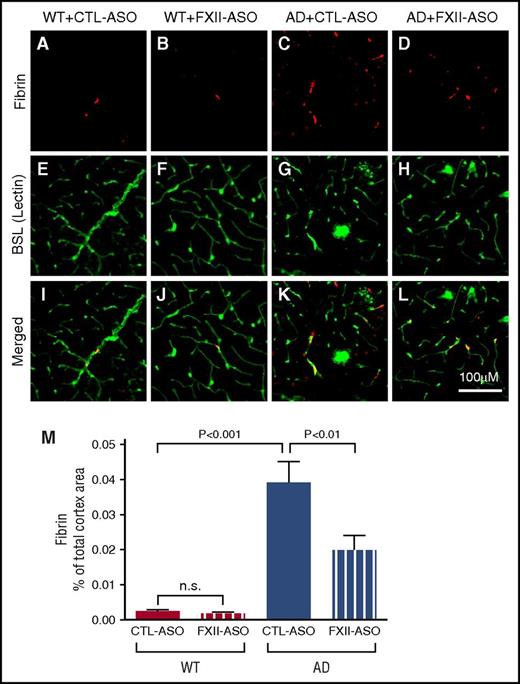
AD is associated with prothrombotic conditions, and microinfarcts and fibrin deposition are increased in AD brains.3,6,10,29 Activation of the plasma contact system can potentially contribute to the prothrombotic conditions in AD by activating the intrinsic coagulation pathway through FXI. To analyze whether activation of the contact system could play a role in this aspect, we compared fibrin deposition in the brains of WT or AD mouse brains treated with CTL-ASO or FXII-ASO (n = 9-14 mice per group) (Figure 5). Blood vessels and microglia/macrophages were stained by lectin. Neither WT group showed fibrin deposition in the brain. Consistent with previous data,30 fibrin deposition in the brains of CTL-ASO–treated AD mice was significantly higher than in WT controls. FXII-ASO–treated AD mice showed significantly reduced fibrin deposition compared with CTL-ASO–treated AD mice. These results suggest that FXII mediates increased fibrin deposition in the AD mouse brain, and depletion of FXII can reduce this deposition.
Fibrin(ogen) deposition is decreased in AD mice treated with FXII-ASO. Brain sections from WT and AD mice treated with CTL-ASO or FXII-ASO were stained with an antibody against fibrin(ogen) (A-D), blood vessels were visualized by lectin staining (E-H), and the images were merged (I-L). CTL-ASO–treated AD mice showed significantly more fibrin(ogen) staining (C) than CTL-ASO–treated WT mice (A). In FXII-ASO–treated AD mice, fibrin(ogen) deposition was significantly reduced (D,L,M) compared with CTL-ASO–treated AD mice (C,K,M). Fibrin(ogen) deposits were minimal in WT mice and were similar between CTL-ASO (A,I,M) and FXII-ASO treatments (B,J,M) (one-way ANOVA; n = 9-14 mice per group). All values presented as mean ± SEM. Results are from 3 independent experiments. Scale bar for panels A-L, 100 μm.
Fibrin(ogen) deposition is decreased in AD mice treated with FXII-ASO. Brain sections from WT and AD mice treated with CTL-ASO or FXII-ASO were stained with an antibody against fibrin(ogen) (A-D), blood vessels were visualized by lectin staining (E-H), and the images were merged (I-L). CTL-ASO–treated AD mice showed significantly more fibrin(ogen) staining (C) than CTL-ASO–treated WT mice (A). In FXII-ASO–treated AD mice, fibrin(ogen) deposition was significantly reduced (D,L,M) compared with CTL-ASO–treated AD mice (C,K,M). Fibrin(ogen) deposits were minimal in WT mice and were similar between CTL-ASO (A,I,M) and FXII-ASO treatments (B,J,M) (one-way ANOVA; n = 9-14 mice per group). All values presented as mean ± SEM. Results are from 3 independent experiments. Scale bar for panels A-L, 100 μm.
Depletion of plasma FXII reduced inflammation-associated neuronal damage in AD mouse brains
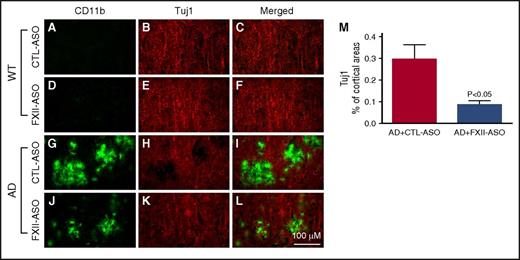
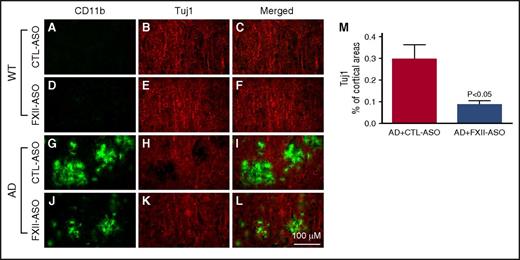
Inflammation and fibrin deposition are associated with neuronal damage and cognitive impairment in AD.30-36 To investigate whether reduced neuroinflammation and fibrin deposition in FXII-ASO–treated AD mice were associated with less neuronal damage compared with CTL-ASO–treated AD mice, we examined the relationship between microglia/macrophage activation and neuronal damage (n = 9-14 mice per group) (Figure 6). CD11b immunohistochemistry was used to indicate microglia/macrophage activation, and Tuj1 immunohistochemistry was used to show neuronal morphology and integrity. In WT mice, there were no detectable changes in microglia/macrophage-associated Tuj1 staining. In the brains of AD mice treated with CTL-ASO, there were regions with robust CD11b immunostaining that correlated with decreased Tuj1 immunostaining, indicating that activated microglia/macrophages were spatially associated with neuronal damage. Treatment of AD mice with FXII-ASO reduced this microglia/macrophage-associated neuronal damage. We also compared NeuN staining (a neuronal marker) among these groups, which revealed that NeuN staining was significantly reduced in CTL-ASO–treated AD mice compared with CTL-ASO–treated WT mice, whereas FXII-ASO treatment significantly restored NeuN staining in AD mice (supplemental Figure 3). These results showed that reduced inflammation in FXII-ASO–treated AD mice is associated with less neuronal damage compared with CTL-ASO–treated AD mice.
Depletion of plasma FXII reduces inflammation-associated neuronal damage in AD mouse brains. Brain sections from CTL-ASO- or FXII-ASO–treated WT and AD mice were stained with antibodies against CD11b (A,D,G,J) and Tuj1 (B,E,H,K), and the images were merged (C,F,I,L). In CTL-ASO–treated AD mice, the staining for Tuj1 was weaker in areas where CD11b staining was robust (G,H,I), indicating microglia/macrophage-associated neuronal damage. This microglia/macrophage-associated neuronal damage was significantly reduced in FXII-ASO–treated AD mice (J-M) compared with CTL-ASO–treated AD mice (G,H,I,M) There were no detectable microglia/macrophage-associated changes in Tuj1 staining in either CTL-ASO- or FXII-ASO–treated WT mice (A-F). Student t test, n = 10/group. All values presented as mean ± SEM. Results are from 3 independent experiments. Scale bar for panels A-L, 100 μm.
Depletion of plasma FXII reduces inflammation-associated neuronal damage in AD mouse brains. Brain sections from CTL-ASO- or FXII-ASO–treated WT and AD mice were stained with antibodies against CD11b (A,D,G,J) and Tuj1 (B,E,H,K), and the images were merged (C,F,I,L). In CTL-ASO–treated AD mice, the staining for Tuj1 was weaker in areas where CD11b staining was robust (G,H,I), indicating microglia/macrophage-associated neuronal damage. This microglia/macrophage-associated neuronal damage was significantly reduced in FXII-ASO–treated AD mice (J-M) compared with CTL-ASO–treated AD mice (G,H,I,M) There were no detectable microglia/macrophage-associated changes in Tuj1 staining in either CTL-ASO- or FXII-ASO–treated WT mice (A-F). Student t test, n = 10/group. All values presented as mean ± SEM. Results are from 3 independent experiments. Scale bar for panels A-L, 100 μm.
Depletion of plasma FXII improved cognitive function in AD mice
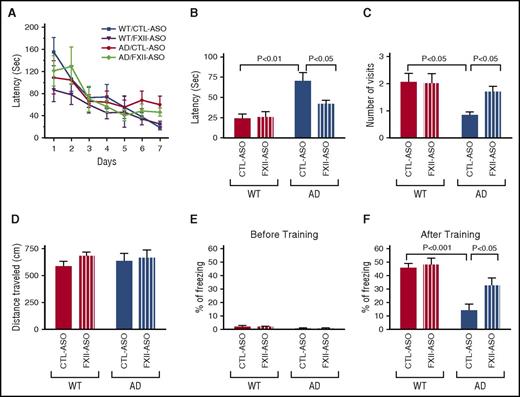
Because fibrin deposition, neuroinflammation, and neuronal damage were reduced in brains of AD mice upon FXII depletion, we analyzed whether these changes were associated with memory improvement. Two-month-old AD mice were treated with CTL-ASO or FXII-ASO for 4 months and then tested for cognitive performance by using the Barnes maze (n = 9-14 mice per group) (Figure 7A-D), which assesses spatial learning and memory in rodents.37 During training, FXII knockdown slightly improved spatial learning in AD mice, but the difference was not significant (Figure 7A). During the memory retention test, CTL-ASO–treated AD mice spent a significantly longer time finding the target hole and had significantly fewer visits to the target hole compared with CTL-ASO–treated WT mice, confirming that memory retention is impaired in AD mice at this age.31 FXII-ASO–treated AD mice spent less time finding the target hole and had significantly more visits to the target hole compared with CTL-ASO–treated AD mice (Figure 7B-C). FXII depletion had no effect on WT mice in these tests. These results showed that treatment of AD mice with FXII-ASO improved their spatial learning and memory.
Depletion of plasma FXII improves cognitive function in AD mice. Spatial learning and memory retention of WT and AD mice were assessed by using the Barnes maze after 4 months of treatment with FXII-ASO or CTL-ASO. (A) During training trials, latency to poke the target hole was measured. (B-D) During the probe trials, latency to reach the closed target hole (B) and number of visits to the target hole (C) were measured. (D) Locomotor function was measured by the total distance traveled during the probe trials (one-way ANOVA; n = 9-14 mice per group). Results are from 3 independent experiments. Cognitive function of WT and AD mice treated with FXII-ASO or CTL-ASO was measured by contextual fear conditioning. (E) Freezing behavior was measured before electric foot shock during the training day to assess the basal freezing tendency of each group of mice. (F) Contextual memory was assessed by measuring freezing behavior upon re-exposure to the training chamber 24 hours after fear conditioning training (two-way ANOVA; n = 9-14 mice per group). Results are from 3 independent experiments.
Depletion of plasma FXII improves cognitive function in AD mice. Spatial learning and memory retention of WT and AD mice were assessed by using the Barnes maze after 4 months of treatment with FXII-ASO or CTL-ASO. (A) During training trials, latency to poke the target hole was measured. (B-D) During the probe trials, latency to reach the closed target hole (B) and number of visits to the target hole (C) were measured. (D) Locomotor function was measured by the total distance traveled during the probe trials (one-way ANOVA; n = 9-14 mice per group). Results are from 3 independent experiments. Cognitive function of WT and AD mice treated with FXII-ASO or CTL-ASO was measured by contextual fear conditioning. (E) Freezing behavior was measured before electric foot shock during the training day to assess the basal freezing tendency of each group of mice. (F) Contextual memory was assessed by measuring freezing behavior upon re-exposure to the training chamber 24 hours after fear conditioning training (two-way ANOVA; n = 9-14 mice per group). Results are from 3 independent experiments.
We examined locomotor function of these mice by measuring the total distance traveled during probe trials. WT and AD mice treated with CTL-ASO and FXII-ASO traveled similar distances during the trials (Figure 7D). This result indicates that the locomotor function of all mouse groups was similar, and the difference in latency and number of target hole visits is a function of memory.
We also tested cognitive function in these mice by using contextual fear conditioning. WT and AD mice treated with CTL-ASO or FXII-ASO had similar baseline freezing behavior (Figure 7E). However, as expected,31 CTL-ASO–treated AD mice showed a memory deficit compared with CTL-ASO–treated WT mice 24 hours after the training (Figure 7F). FXII-ASO–treated AD mice showed a significant improvement in memory when compared with CTL-ASO–treated AD mice (Figure 7F). This result corroborates the results from the Barnes maze and shows that depletion of FXII can ameliorate the cognitive deficit in AD mice.
Discussion
Previous studies have shown that Aβ can activate the plasma contact system both in vitro and in vivo.17-20,38-40 In AD patient plasma, levels of activated FXII, HK cleavage, and kallikrein activity are increased compared with control plasma.19 Moreover, cleavage of fibrinogen, a major clot protein, is also increased in AD patient plasma, indicating activation of the coagulation pathway.20 Consistent with the increased fibrinogen cleavage, levels of FXI are decreased in AD patient plasma,20 suggesting contact system–dependent consumption of FXI. These results suggest that both coagulation and inflammatory pathways of the contact system are activated in AD patient plasma. AD patients show increased incidence of brain microinfarcts,11,41 stroke,4 fibrin deposition, and inflammation in the brain,30,33,42-45 all of which are consistent with a role for contact system activation in AD pathology.
In two different mouse models of AD, Tg679946 and TgCRND8,22 in which AD pathology is driven by the overexpression of human Aβ, HK cleavage is increased (Figure 1).19 Injection of human Aβ peptide into WT mice also increased HK cleavage,19 indicating that Aβ is likely an activator of the contact system in vivo. In this study, we showed that HKi levels in AD mice were significantly lower than those in WT mice. After FXII knockdown, HKi levels in AD mice were higher than those in control AD mice (Figure 2), indicating that FXII mediates HK cleavage in AD mice. AD mice also have increased blood clotting and fibrin deposition in the brain,30,31,33 and reducing fibrinogen levels in these AD mice improved their cognitive abilities.33 The results presented here show that depletion of FXII in AD mice reduced fibrin deposition and ameliorated their cognitive decline (Figures 5 and 7). Our previous study showed that Aβ42-mediated thrombin generation is FXII dependent but extrinsic coagulation pathway independent,20 and therefore increased fibrin deposition in the AD brain is likely mediated by FXII. Taken together, these results from AD mouse models support a role for contact system activation in AD pathogenesis.
Inflammation plays an important role in AD pathology. Microglia and astrocytes are activated and found surrounding Aβ plaques in postmortem brain tissue of AD patients. Cytokine expression is also increased in AD brains.14,15 In many AD mouse models, inflammation is evident with robust microglial and astrocyte activation.15,31,44 Aβ deposition may play an important role in neuroinflammation in the AD brain, but other factors may also contribute.47
Bradykinin, released by activation of the contact system, is an inflammatory mediator.48 It can increase vascular permeability, leading to vascular leakage and angioedema.49 Bradykinin is involved in inflammatory responses in the peripheral and central nervous systems through the activation of 2 receptors, B1R and B2R. B2R is constitutively expressed in various tissues and cell types, whereas B1R is induced upon stimulation or tissue damage.50,51
Bradykinin and its receptors may play important roles in AD pathology and AD-associated cognitive impairment. B2R is expressed in the cerebrovascular endothelial cells, whereas B1R expression is increased in astrocytes, neurons, and vascular cells of AD mice.52,53 Infusion of the Aβ peptide into the rat brain increases bradykinin levels in the cerebrospinal fluid and B1R expression in brain regions related to memory.54 Moreover, this phenomenon is accompanied by memory disruption and neuronal loss.
The increase in contact system activation in plasma of both AD patients and AD mice19,20,40 could result in increased bradykinin release due to HK cleavage, which could in turn activate B2R on the cerebrovascular endothelial cells and increase blood brain barrier (BBB) permeability.44,45,55-58 Our results show that fibrin deposition in FXII-ASO–treated AD mice was reduced, indicating that activation of the contact system may contribute to increased BBB permeability in AD, whereas FXII-ASO treatment protects against vascular leakage (Figure 5). A compromised BBB in AD would allow plasma proteins, including components of the contact system and bradykinin, to leak into the brain. Contact system proteins in the brain parenchyma could be activated locally by Aβ,17-20,38-40 which is highly concentrated and deposited in the brain parenchyma. Indeed, in postmortem AD patient brain sections, researchers have shown that FXII codeposits with parenchymal Aβ plaques.59 Cerebrospinal fluid from AD patients has increased contact system activation and HK cleavage,21 indicating that the contact system is activated in the brains of AD patients.49
Activation of the contact system in the brain would result in bradykinin production, which could activate B1R in parenchymal cells, such as astrocytes, to promote neuroinflammation. B1R antagonist treatment of an APP transgenic AD mouse model improved spatial learning.52 Although B1R is mostly expressed in astrocytes in the AD mouse model, the B1R antagonist reduced microglial activation, indicating that B1R may mediate astrocyte regulation of microglial activation in AD.52 In the TgSwDI AD mouse model, blockage of B1R also decreases accumulation of activated microglia and reactive astrocytes, diminishes NF-kB activation, and reduces cytokine and chemokine levels in the brain.53 In addition, genetic deletion of B1R also improves cognitive deficits in a mouse model of AD.60 These results further support that bradykinin receptors play a role in AD. Our results showed that contact system activation is temporally correlated with astrocyte activation in the AD mouse brain. ASO-mediated depletion of FXII in AD mouse plasma diminished HK cleavage (Figure 2), which would inhibit bradykinin release, and resulted in reduced astrocyte and microglia/macrophage activation (Figures 3 and 4), suggesting that the plasma contact system may regulate brain inflammation in AD at least in part through B1R.
In addition, fibrin deposition in the central nervous system61-63 and in the AD brain is known to cause inflammation.30,33,44 Because depletion of FXII reduced fibrin deposition in the AD mouse brain, the reduced brain inflammation in FXII-ASO–treated AD mice could be a combination of a functional change of bradykinin and reduced fibrin deposition.
On the basis of our studies, we hypothesize that increased FXII activation in AD patient and AD mouse plasma converts PPK to kallikrein, which cleaves HKi to release bradykinin. Elevated plasma bradykinin may increase BBB permeability leading to plasma bradykinin leakage into the brain which, in turn, could activate bradykinin receptors in the brain and promote neuroinflammation and neuronal damage. Furthermore, a compromised BBB in AD would allow plasma contact system proteins to leak into the brain parenchyma where they could be activated by Aβ, further increasing bradykinin receptor activation and inflammation. In addition to these effects, increased FXII activation can initiate the intrinsic coagulation pathway via FXI, leading to clot formation, vascular occlusion (hypoperfusion), and fibrin deposition (increased BBB permeability in AD can also contribute to fibrin deposition), which is known to trigger neuroinflammation.61 As a result, activation of both inflammatory and coagulation pathways could ultimately promote neurodegeneration and cognitive decline (supplemental Figure 4).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Daria Zamolodchikov for indispensable discussions throughout this work, Odella C. Jno-Charles and Allison Richards for experimental assistance, and Hyung Jin Ahn, Marta Cortes-Canteli, and members of the Strickland laboratory for their help.
This work was supported by National Institutes of Health, National Institute of Neurological Disorders and Stroke Grant No. NS050537 (S.S.), Cure Alzheimer’s Fund, Rudin Family Foundation, Mellam Family Foundation, Louis Herlands, John A. Herrmann Jr, and Mary and James G. Wallach Foundation.
Authorship
Contribution: Z.-L.C. designed the study, performed experiments, analyzed data, and wrote the manuscript; A.S.R. designed the study and participated in data analysis and manuscript preparation; P.S. performed experiments and analyzed data; A.R.M. participated in data analysis and manuscript preparation; and E.H.N. and S.S. designed the study and participated in data analysis and manuscript preparation.
Conflict-of-interest disclosure: A.S.R. and A.R.M. are employees and stockholders of Ionis Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Sidney Strickland, Patricia and John Rosenwald Laboratory of Neurobiology and Genetics, The Rockefeller University, 1230 York Ave, New York, NY 10065; e-mail: strickland@rockefeller.edu.