Oral anticoagulant therapy for venous thromboembolism is very effective. When oral anticoagulants are managed well, the risk of recurrence is approximately 2 per 100 patient-years. The main reasons for a breakthrough event are underlying disease and subtherapeutic drug levels. The most common underlying disease that results in recurrence on treatment is cancer. Subtherapeutic drug levels can be caused by poor adherence to the drug regimen, interactions with other drugs or food, or inappropriate dosing. It is important to investigate and understand the cause whenever such an event occurs and to improve management of anticoagulants thereby avoiding further recurrences. Here we present 4 illustrative cases together with a discussion of the underlying pathology. Whereas the mechanisms are usually quite well understood, the management of further anticoagulation after a breakthrough event is based on minimal or no clinical trial evidence.
Introduction
Risk of recurrence in different populations
Vitamin K antagonists (VKAs) have a long track record of providing effective protection against recurrences after venous thromboembolism (VTE). In a Cochrane meta-analysis of studies comparing shorter versus longer duration of treatment with VKAs, the risk of recurrence in the longer duration of treatment arm was 1.6%.1 These studies generally excluded patients with known cancer. In 5 randomized trials in patients with cancer, 82 (14%) of 571 patients treated with VKAs had recurrent VTEs vs 42 (7.1%) of 591 patients receiving low-molecular-weight heparin (LMWH) during the first 3 to 6 months of treatment.2
The non-vitamin K antagonist oral anticoagulants (NOACs) have not shown superiority to VKAs regarding efficacy in the treatment of VTEs. In the treatment of acute VTEs, the relative risk for recurrence with NOACs vs VKAs was 0.85 (95% confidence interval [CI], 0.55-1.31).3 On the basis of subgroup analyses from some of these studies, the corresponding relative risk in patients with cancer was 0.77 (95% CI, 0.44-1.33).4 However, this was mainly in cancers without metastases and not with LMWH as a comparator. Results from clinical practice studies also show similar efficacy for rivaroxaban vs VKAs, rivaroxaban being the only NOAC that has been approved for a long enough time to allow for such observations. Thus, in the international Xarelto for Long-Term and Initial Anticoagulation in Venous Thromboembolism (XALIA) cohort study, the adjusted hazard ratio for VTE recurrence with rivaroxaban vs VKAs was 0.91 (95% CI, 0.54-1.54)5 and in the Swiss Venous Thromboembolism Registry (SWIVTER), it was 0.55 (95% CI, 0.18-1.65).6
New symptoms of deep vein thrombosis or pulmonary embolism are not proof of recurrent events, even when there seems to be support from diagnostic imaging. In the RE-COVER study7 comparing dabigatran with warfarin for the treatment of acute VTE, all patients at baseline had ultrasonography of both legs and computed tomography (CT) or ventilation-perfusion scanning of the lungs whether they had symptoms or not. Central adjudication of locally suspected recurrences on diagnostic imaging, using comparisons with baseline data, did not confirm suspected recurrent pulmonary embolism in 7.5% and did not confirm suspected recurrent deep vein thrombosis in 11.8% (unpublished data). Ipsilateral recurrence of thrombosis poses a special diagnostic challenge. When there is a questionable difference in extension of the VTE between the images from the first event and the recurrence, a negative D-dimer in a patient with onset of symptoms within the last few days speaks against a true recurrence.
Patients admitted to the hospital with a new diagnosis of pulmonary embolism while already receiving warfarin compared with those admitted with pulmonary embolism who are not receiving anticoagulation, have a 4.4-fold higher risk of fatal recurrent pulmonary embolism after discharge from the hospital.8 These deaths are mainly related to cardiovascular disease, malignancy, and sepsis.
Causes for breakthrough thromboembolism
Underlying condition or disease.
There are many potential causes for recurrence of VTE despite anticoagulant therapy. From the above-mentioned trial data, it is obvious that presence of cancer increases the risk of recurrence. In the largest registry of VTE treatment in clinical practice, Registro Informatizado de la Enfermedad Trombo Embolica (RIETE), every third patient with recurrence who was receiving VKA therapy had cancer.9 This is the cardinal example of an underlying disease exhibiting pronounced thrombogenicity that overcomes the protective effect of anticoagulants. The malignant tumors exert this effect through several mechanisms that, among others, involve activation of coagulation and obstruction of blood flow and have been extensively reviewed elsewhere.10 For patients with myeloproliferative neoplasms (polycythemia vera and essential thrombocythemia), molecular profiling with analysis of JAK2, CALR, and MPL mutations can add information on the risk of thrombosis that often occurs in atypical sites.11 Diseases or conditions with an increased risk of recurrent VTE on anticoagulation are summarized in Table 1. The mechanism behind the increased thrombogenicity is sometimes unclear, such as in Behçet disease and antiphospholipid syndrome, and many alternatives have been proposed (Table 1). However, for heparin-induced thrombocytopenia, the pathogenesis has been explained: immunoglobulin G antibodies that recognize the multimolecular complexes between heparin and platelet factor 4 assemble on the surface of platelets that become activated and release procoagulant microparticles.12
A minority of patients with antiphospholipid syndrome display a falsely elevated international normalized ratio (INR), possibly because of antibodies against tissue factor, and they may thus have recurrent events despite optimal INRs.13 They would typically have demonstrated a prolonged prothrombin time before starting treatment with an anticoagulant, and alternative thromboplastins that are insensitive to the lupus anticoagulant should then be used for monitoring warfarin therapy.14 INR results from point-of-care instruments are variable and not reliable for patients with lupus anticoagulant.
Paroxysmal nocturnal hemoglobinuria, diagnosed in 1 per 100 000 people per year15 and caused by the expansion of an abnormal hematopoietic clone, is characterized by intravascular hemolysis, cytopenias, and thrombosis. The latter often occur in unusual sites (intra-abdominal or intracranial veins), and treatment failures in patients receiving anticoagulant therapy have been reported at 10.6 events per 100 patient-years.16 In 1 study, 9 of 41 patients with thrombosis experienced recurrences when receiving anticoagulants.17 A concomitant bleeding tendency often makes it difficult to increase the anticoagulant intensity, and targeting of the terminal complement activation complex by the addition of eculizumab is probably the best solution.18,19
During pregnancy, many changes occur in the prohemostatic direction, essentially preparing the woman for childbirth without massive bleeding but with a concomitant increase in risk of thrombosis (Table 1). Furthermore, the presence of the antiphospholipid syndrome will increase the risk of gestational venous thromboembolism as well as unexplained fetal death, spontaneous abortions, and premature birth.20 In the RIETE registry with 607 women experiencing VTEs during pregnancy or puerperium, 16 had a recurrence and 11 of those occurred during treatment with anticoagulants.21 However, only 1 of the patients with recurrence who was receiving anticoagulation had antiphospholipid syndrome.
Thrombophilia, other than antiphospholipid syndrome, is sometimes found in patients with breakthrough events, but there is no convincing evidence that such events are more common in patients with hereditary thrombophilic defects.22 It has been speculated that deficiency of antithrombin may be associated with heparin resistance. In a retrospective review of 70 patients with congenital antithrombin deficiency, we found 8 patients in whom there was some clinical deterioration on treatment with unfractionated heparin.23
Finally, vascular anomalies with chronic obstruction of the venous flow may cause recurrences while the patient (typically young patients) is receiving anticoagulation. These abnormalities include the thoracic outlet syndrome and May-Thurner syndrome (compression of the left common iliac vein by the right iliac artery).24 There are several options for managing these thrombotic events, including initial thrombolysis, pharmacomechanical clot removal, endovascular stenting, or decompressive surgery (thoracic outlet syndrome).25
Inappropriate dosing of anticoagulant.
For patients treated with VKAs, it is crucial to remember that it takes at least 5 days for all the vitamin K–dependent coagulation factors to decrease to sufficiently low activity to provide a therapeutic anticoagulant effect. Therefore, overlap with a parenteral anticoagulant for at least 5 days and until the INR has reached 2.0 for at least 24 hours is recommended (Grade 1B, explained in Table 2).26
It may take 4 to 6 weeks until treatment with a VKA results in stable therapeutic INRs (ie, maintaining the range of 2.0-3.0). Many patients will have 1 or several subtherapeutic INRs during this first treatment phase, which could explain the frontloaded trend to superior efficacy of NOACs seen in the studies with rivaroxaban in deep vein thrombosis27 and with apixaban in VTE.28
Patients receiving anticoagulation therapy with VKAs and the physicians managing them need to be aware of the large number of drug-drug interactions that can affect the INR levels and that require dose adjustments. This often becomes demanding when patients start treatment with an interacting drug that has a slow, progressive interaction that requires repeated dose adjustments over weeks or even months. An example of this is rifampicin, which causes a delayed induction of the microsomal enzyme CYP2C9, which is responsible for most of the metabolism of warfarin.29 A reverse example is amiodarone, a general inhibitor of cytochrome P450–catalyzed oxidation, thereby decreasing the effect of CYP2C9.30 When amiodarone is discontinued, there is a delayed increase in dose requirements to maintain therapeutic warfarin effect. In both cases, frequent monitoring of anticoagulation during several months is required to avoid suboptimal dosing and recurrent VTE.
The importance of drug-food interactions with VKAs involving the vitamin K–rich dark green vegetables has often been exaggerated to the extent that some patients stopped eating any vegetables when taking warfarin. Patients should be encouraged to maintain a consistent, healthy diet. However, if the patient decides to embark on a vegetarian diet, there might be a need to increase the dose of VKAs. Avocado was reported to antagonize warfarin, perhaps more from the oil inhibiting warfarin absorption than via vitamin K.31
The NOACs definitely have fewer drug-drug interactions than VKAs. Concomitant use of rifampicin, phenytoin, carbamazepine, St. John’s wort, and possibly phenobarbital will cause strong induction of CYP3A4. This leads to a decrease of the area under the curve (ie, exposure by 50% or more for rivaroxaban and apixaban, as described in the respective product monographs). Edoxaban is much less dependent on CYP3A4 metabolism, but it is a substrate for the P-glycoprotein (P-gp) efflux transporter like all other NOACs, and rifampicin will cause a 33% increase in the clearance of edoxaban.32 Dabigatran is not metabolized via the CYP3A4 pathway, but again, it is a substrate for P-gp. Concomitant use of rifampicin or any of the other strong CYP3A4 or P-gp inducers is therefore not recommended with any of the NOACs.
Thus, one of the above-mentioned interactions could be the explanation for a VTE recurrence when a patient is receiving anticoagulation therapy with a NOAC, and the current medication list should be carefully reviewed before prescribing an alternative oral anticoagulant or intensifying current anticoagulation therapy. For rivaroxaban, there is an additional caveat. At the 15- to 20-mg doses used for treatment of VTE (as opposed to a 10-mg dose used for VTE prophylaxis), rivaroxaban depends on the presence of food for optimal intestinal absorption, as already reported from studies in healthy volunteers.33 Exposure to rivaroxaban taken without food might be even lower in patients than in healthy volunteers, as described in a patient with recurrent pulmonary embolism who was receiving rivaroxaban.34 In my clinical practice, I have identified several patients with recurrent VTE or cardioembolic stroke under the same circumstances.
Another scenario with suboptimal exposure is the morbidly obese patient. There are no recommendations for dose adjustments of NOACs for this population, which was underrepresented in the clinical trials.
Patients with major gastrointestinal tract surgery might have reduced absorption of a NOAC. In a review of the literature, only case series or single case reports were identified.35 It seemed that reduced anticoagulant efficacy could be experienced with dabigatran, and possibly also with rivaroxaban after Roux-en-Y gastric bypass or after gastrectomy. There was no information regarding the absorption of apixaban or edoxaban in such patients.
In the setting of stroke prophylaxis in atrial fibrillation, it has been shown that patients are more stroke averse than physicians but physicians are more bleeding averse than patients, which leads to underdosing.36 The NOACs are typically labeled for a standard dose and a reduced dose regimen, according to specific criteria for stroke prophylaxis in atrial fibrillation and in some countries for VTE. Retrospective medical record reviews have shown a high degree of inappropriate dose selection, even in hospitals,37,38 with many of those patients receiving a suboptimal dose. This may also be true for the patients with VTE and is thus another explanation for breakthrough events. The approved dose regimens for treatment of VTE with NOACs differ somewhat between the United States and other countries. For example, dabigatran is only approved at a dose of 150 mg twice per day in the United States; in other countries, the drug label has a dose reduction to 110 mg twice per day for patients older than age 80 years or older than age 75 years if they have an increased risk of bleeding. This dose reduction is not based on data from the studies of treatment in VTE, and only observational studies will confirm if this dose is sufficiently effective.39 There are also criteria based on low body weight, impaired renal function, and/or older age for reduction of the dose of rivaroxaban or apixaban in patients with atrial fibrillation but not in patients with VTE, which is bound to result in confusion. Conversely, for edoxaban, the dose reduction is recommended for both indications for patients with moderate renal impairment, low body weight, and/or concomitant use of P-gp inhibitors (Table 3).
Evaluation and investigation of the patient
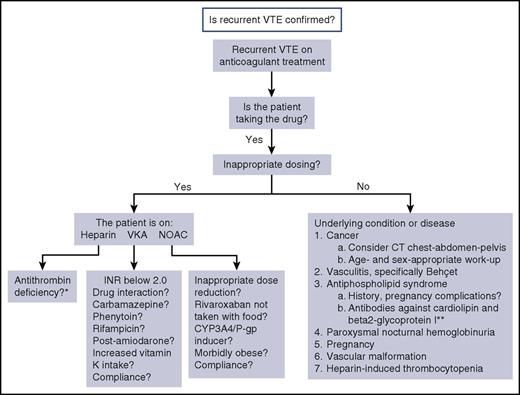
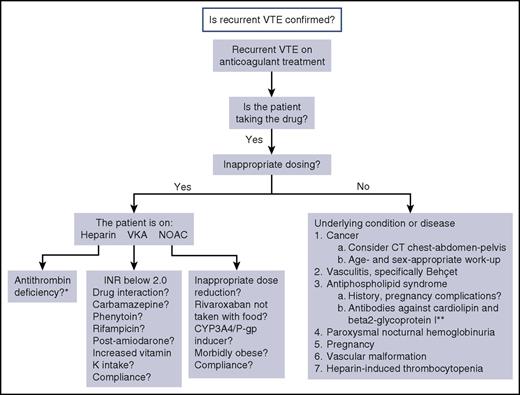
A suggested algorithm for evaluating the cause for recurrent VTE in a patient who is receiving anticoagulation therapy is shown in Figure 1. Rare causes for breakthrough events may have been missed. Further details regarding evaluation and management will be discussed in association with the case examples below. The reason for the therapeutic failure may not be apparent at the first encounter with the patient. Although investigations are continuing, the most appropriate and effective anticoagulant therapy should be provided. If a malignancy is highly suspected but not yet proven, it would be appropriate to treat according to that hypothesis (see Case 1). In general, LMWH at a therapeutic dose, or sometimes higher, is a good option unless heparin-induced thrombocytopenia is a possibility.
Suggested algorithm for investigation of cause for recurrent VTE on anticoagulation. *Antithrombin level becomes falsely low during treatment with heparin. **Testing for lupus anticoagulant is not possible while the patient is receiving any anticoagulant.
Suggested algorithm for investigation of cause for recurrent VTE on anticoagulation. *Antithrombin level becomes falsely low during treatment with heparin. **Testing for lupus anticoagulant is not possible while the patient is receiving any anticoagulant.
Some investigations are not feasible or are fraught with misinterpretation for patients who are receiving anticoagulant treatment. For example, the antithrombin level is reduced during treatment with heparin. Lupus anticoagulant testing may become false positive on any anticoagulant treatment, but it is not advisable to hold treatment to obtain this test in view of the very hypercoagulable state of the patient.
Case 1: cancer and recurrent thrombosis
A 56-year-old man was diagnosed with proximal deep vein thrombosis in the right leg, confirmed with compression ultrasonography. He was diagnosed with an unprovoked thrombosis in the left leg 3 months earlier and has since been treated with warfarin after an initial overlap with LMWH. His INR is now 2.9 and has been in the therapeutic range most of the time. The patient also complains of upper abdominal pain and poor appetite and has lost about 5 kg since the last visit.
Initial investigation and anticoagulation
We suspected a malignancy and ordered a CT scan of the abdomen and pelvis. In the SOMIT study, this was the investigation with the highest yield in the group allocated to extensive screening for cancer.40 While awaiting the CT result, we stopped warfarin and started LMWH at a therapeutic dose. The CT scan demonstrated cancer of the pancreas with liver metastases. Therefore, the patient will continue on LMWH at a therapeutic dose rather than going back to an oral anticoagulant for now. This is in accordance with the recommendations by guidelines from the American College of Chest Physicians (ACCP) (Grade 2C),41 a guidance document supported by the International Society on Thrombosis and Haemostasis (ISTH),42 a guidance document by Anticoagulation FORUM,43 and an informal suggestion from the American Society of Clinical Oncology (ASCO).44 The recommendations or suggestions are based on the superior efficacy of LMWH compared with VKAs in patients with cancer and mainly a first event of VTE. An ISTH registry of patients with cancer and recurrent VTE who were receiving anticoagulation therapy confirmed that these patients also experienced fewer recurrences over the next 3 months on LMWH vs warfarin (hazard ratio, 0.28; 95% CI, 0.11-0.70).45 Nevertheless, additional recurrences in patients receiving anticoagulation are not uncommon: 11% during 3 months in the ISTH registry.45
Beyond the acute phase
The CLOT study used a protocol with the LMWH dalteparin at full therapeutic dose for 1 month, followed by 75% of the dose for the next 2 to 5 months.46 In the CATCH study, patients in the LMWH arm received tinzaparin at full therapeutic dose for the entire 6 months.47 The guideline and guidance documents do not provide any suggestions regarding dose reduction after a certain number of months.41,42,44 This has left me to choose between maintaining the dose at 100% or reducing the dose to 75% after 1 month, depending on the perceived risk of recurrence (thrombogenic tumor type, extensive thromboembolism) and risk of bleeding.
For patients with a calculated creatinine clearance below 30 mL/min, corresponding to severe renal impairment, the dose of enoxaparin should be reduced to 1 mg/kg once per day. The product monographs for dalteparin and tinzaparin recommend that for patients with severe renal impairment, a dose reduction should be considered, but they do not specify how much the dose should be reduced. Nadroparin is contraindicated in severe renal impairment.
Recurrent VTE in patients receiving a therapeutic dose of LMWH
In a retrospective study of patients with cancer and with recurrent VTE who were receiving anticoagulation therapy, 47 patients who were already receiving treatment with a therapeutic dose of LMWH had dose escalation of 20% to 25% for 4 weeks.48 Some patients were receiving a prophylactic dose and others were receiving a therapeutic dose of LMWH when the breakthrough event happened. Only the latter subset of patients was escalated to a supratherapeutic LMWH dose of about 120%. The recurrence rate beyond this dose escalation was 8.6% during 3 months, with 3 patients each receiving a therapeutic dose or 120% of the therapeutic dose.48 In the international ISTH registry, there was no significant difference in the risk of further recurrences over 3 months between patients who had a dose escalation of ≥20% (or ≥25%) and those with an unchanged dose of LMWH.45 The ACCP guidelines recommend a dose escalation of LMWH by one-quarter to one-third (Grade 2C),41 and ASCO recommends a 20% to 25% increase (no grade).44
In clinical practice, it is often difficult to maintain patients on a twice-per-day injection regimen for prolonged periods. After a recurrence on enoxaparin at 1.5 mg/kg per day, a patient could change to either enoxaparin 1 mg/kg twice per day or, for patients who are not overweight, to tinzaparin at slightly more than 200 units/kg once per day, which might fit into one prefilled syringe. Insertion of a vena cava filter is not recommended for these patients unless there is a contraindication to anticoagulation or if there is recurrent pulmonary embolism when the patient is receiving adequate anticoagulation.42
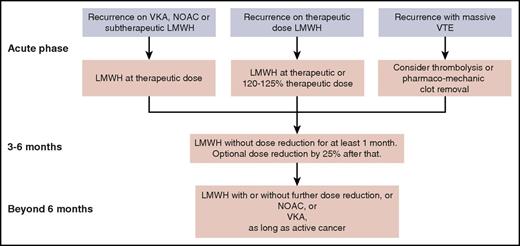
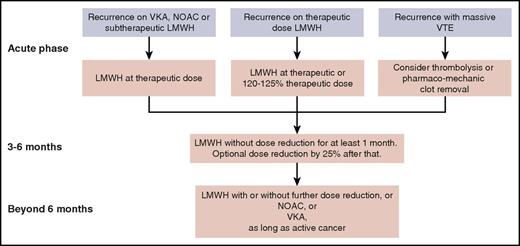
The NOACs are not yet recommended for initial treatment in the absence of any study comparing such a drug head-to-head with LMWH. In the near future, results are expected from such a study comparing edoxaban with LMWH,49 and another study comparing apixaban with LMWH (NCT03045406). After the first 3 to 6 months of treatment, patients who still have active cancer continue to have a high risk of recurrent VTE. It is unlikely that we will see a study comparing different anticoagulants for this extended phase.50 Here, NOACs are a valid option in view of the data from a meta-analysis of subgroups from the phase 3 trials with NOACs,4 but VKAs or, for patients who still tolerate it, LMWH is another option. Treatment suggestions for recurrent VTEs in patients with cancer who are receiving anticoagulation therapy are summarized in Figure 2. Decisions on dose reductions should take into account the risk of bleeding and renal function.
Suggested management of recurrent VTE for a patient with cancer who is receiving anticoagulation therapy, from the acute phase through the intermediate- and long-term phases.
Suggested management of recurrent VTE for a patient with cancer who is receiving anticoagulation therapy, from the acute phase through the intermediate- and long-term phases.
Case 2: antiphospholipid syndrome
A 33-year-old woman is seen in the emergency room for acute shortness of breath and fatigue. She has a history of recurrent spontaneous abortions, and investigation has demonstrated that she is positive for lupus anticoagulant and antibodies against cardiolipin. She has recently had a successful pregnancy, managed with LMWH and aspirin throughout and until 6 weeks postpartum. At 3 months postpartum, she developed deep vein thrombosis in the right leg and was treated initially with LMWH, overlapping to warfarin. The patient has continued breastfeeding the baby. She is now 5 months postpartum and her INRs have been difficult to maintain between 2.0 and 3.0, now being 1.6, a typical finding in patients with the antiphospholipid syndrome.51 Ventilation-perfusion lung scanning demonstrated bilateral segmental defects on the perfusion but not on the ventilation scan; thus, there is a high probability for pulmonary embolism.
Treatment should now be switched back to LMWH at a therapeutic dose for 1 week. Once the symptoms have abated, the dose can be reduced to a prophylactic level (eg, dalteparin 5000-7500 IU once per day or enoxaparin 40-60 mg once per day. In a systematic review, long-term anticoagulation with LMWH was similarly effective and had a lower risk of bleeding than a VKA.52 Although several of the 15 included studies used a therapeutic dose of LMWH in the long term, there were similar results for efficacy after pooling the 6 studies with a prophylactic dose: odds ratio for recurrent VTE of 0.91 (95% CI, 0.50-1.60) for LMWH vs VKA. In a case series of 24 patients with antiphospholipid syndrome and thrombosis, long-term secondary prophylaxis was given with dalteparin 5000 units per day for a mean of 309 days, and only 1 treatment failure was reported.53 For patients like the one in this example with a recurrence on VKA, I would give the higher prophylactic dose, that is, dalteparin 7500 IU or enoxaparin 60 mg per day over the long term.
One of the NOACs could be a more convenient alternative for this patient once she stops breastfeeding, and there are emerging data from case series54 and subgroup analyses from larger trials22 that NOACs are similarly effective in patients with antiphospholipid syndrome. However, until a current randomized trial has demonstrated efficacy results,55 NOACs should not be the first line of treatment because failures have been reported.56,57
Case 3: suspected Behçet disease
A 39-year-old man, an immigrant from Syria, was diagnosed with deep vein thrombosis in the popliteal vein of the right leg 2 months ago. It was apparently unprovoked, and he was initially treated with LMWH overlapping with warfarin. During the last month, his INRs have been between 2.0 and 3.0. He now has more pain in the right thigh and the calf is more swollen. Ultrasonography confirms progression of the thrombus up to the mid-femoral vein. He has not lost any weight, has a good appetite, and has normal bowel function. Five years ago, in his home country, he had some kind of inflammation in 1 eye. Upon further questioning, he admits to having had some ulcers in his mouth that he thought were cold sores. On physical examination, there are no current mouth sores or genital sores but there is acne. Visual acuity is decreased in the eye that had the inflammation.
Behçet disease is suspected because the patient seems to fulfill the obligatory criterion of mouth sores and 2 of the additional 4 signs required (possible uveitis and acne but not genital sores and no pathergy test yet). The pathology of venous thrombosis in Behçet disease is mainly attributed to inflammation of the vessel wall, and the thrombi become very adherent with a low risk for pulmonary embolism.58 Anticoagulants have not provided good protection against recurrences in reported cases.59 In the 2008 recommendations of the European League Against Rheumatism (EULAR) for management of Behçet disease, anticoagulation received the weakest strength of recommendation (D) based on the lowest level of evidence (IV).58 Instead, immunosuppressive agents are recommended (Table 4). The monoclonal antibody against tumor necrosis factor α, infliximab, has more recently been used with success in some patients with venous thrombosis.60,61 I have used colchicine with good effect in a patient with anticoagulant-refractory deep vein thrombosis, and similar experiences have been published.62
Case 4: recurrence on treatment with a NOAC
A 42-year-old man was previously healthy until he suffered an unprovoked deep vein thrombosis in the left leg 4 months ago. Compression ultrasonography showed lack of compressibility from the mid-femoral vein to the trifurcation. He began treatment with rivaroxaban 15 mg twice per day, switching after 3 weeks to rivaroxaban 20 mg per day. During the past 4 days, he has experienced increasing pain in the same leg and has difficulty walking. On physical examination, the left leg is 3 cm larger than the right at the maximum calf level and 2 cm larger at the ankle, showing slight reddish discoloration but no pitting edema. His body weight is 142 kg. Repeat ultrasonography shows extension of the thrombosis to the proximal femoral and the common femoral vein.
The patient has not had any weight loss, and review of systems is negative. He works as a taxi driver. The patient claims that he is taking rivaroxaban diligently once per day, and his pharmacy verifies that he renewed his prescription on the expected date. However, he admits that he does not always have time to eat breakfast in the morning when he takes his medication. Thus, we have a patient who seems to be compliant, but his body weight is at the extreme end of the population in the clinical trials (14% weighed >100 kg), and the absorption of the drug has been suboptimal part of the time. Pharmacokinetic results from human volunteers showed that the peak concentration of rivaroxaban was unaffected in patients who weighed >120 kg,63 but only 12 patients had a body weight >100 kg. A guidance document from ISTH on the use of NOACs for obese patients suggests that these drugs should not be used for patients with a body weight >120 kg.64 It also suggests that if a NOAC is used anyway, drug-specific levels at peak and trough should be obtained. Pharmacokinetic studies in healthy volunteers also showed that the absorption of the 20-mg dose of rivaroxaban taken on a fasting stomach is 66% of the absorption when rivaroxaban is taken with food33 and, in patients, the difference might be greater.34
We obtained a rivaroxaban-calibrated drug level 3 hours after the patient took a dose with food and it was 55 ng/mL, which is quite low. Thus, it is reasonable to follow the ISTH guidance document64 and abandon further treatment with a NOAC and continue instead with warfarin, overlapping initially with LMWH.
Conclusion
Recurrent VTE for patients who are receiving anticoagulation therapy is not very common, and new symptoms from the leg could be a manifestation of postthrombotic syndrome. There are several differential diagnoses for chest pain or shortness of breath. When a recurrence is confirmed by differences in VTE extent compared with the diagnostic imaging before anticoagulation therapy was started, the cause should be carefully investigated, because the management differs. The first step is to identify inappropriate anticoagulation therapy (ie, incorrect dose, poor adherence, or drug interactions that may reduce the anticoagulant effect). Once any of these have been excluded, the investigation should be focused on diseases or conditions with hypercoagulability, of which cancer is the most common. The recommendations regarding continued management are usually weak because of the low-quality level of evidence.
Authorship
Contribution: S.S. collected all the information and prepared the manuscript and figures.
Conflict-of-interest disclosure: S.S. has grant support/honoraria from Boehringer Ingelheim, Octapharma, Baxter, Bayer Healthcare, Sanofi, and Bristol-Myers Squibb.
Correspondence: Sam Schulman, Thrombosis Service, Hamilton Health Sciences–General Hospital, 237 Barton St East, Hamilton, ON L8L 2X2, Canada; e-mail: schulms@mcmaster.ca.