In this issue of Blood, Ueda et al demonstrate that a mutation in factor H (FH) disrupts host cell interactions, leading to dysregulation of complement, systemic thrombotic angiopathy, and hemolytic uremic syndrome.1
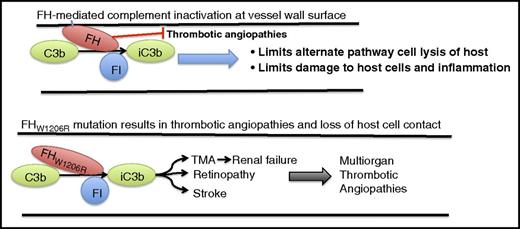
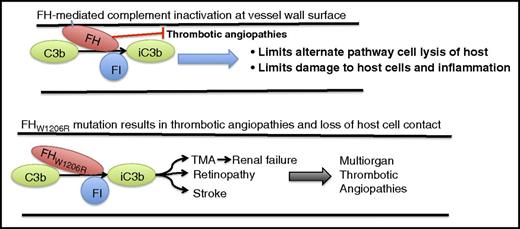
FH regulation of complement involves interacting with the host cell on the carboxyl terminal domains and interacting with complement C3b and FI on the amino terminal of the protein. This interaction results in inactivation of C3b (iC3b), prevention of host cell lysis and inflammation, and prevention of complement-mediated micro- and macrothrombotic angiopathies. A mutation on the carboxyl end of FH at W1206R (W1183R in human) results in a loss of host cell contact by FH without affecting regulation of complement. This loss of host cell interaction appears to result in a multiorgan thrombotic angiopathy, including stroke, retinopathy, and TMA, which leads to renal failure.
FH regulation of complement involves interacting with the host cell on the carboxyl terminal domains and interacting with complement C3b and FI on the amino terminal of the protein. This interaction results in inactivation of C3b (iC3b), prevention of host cell lysis and inflammation, and prevention of complement-mediated micro- and macrothrombotic angiopathies. A mutation on the carboxyl end of FH at W1206R (W1183R in human) results in a loss of host cell contact by FH without affecting regulation of complement. This loss of host cell interaction appears to result in a multiorgan thrombotic angiopathy, including stroke, retinopathy, and TMA, which leads to renal failure.
Complement is an innate system designed in nature to limit and fight infection through antibody-mediated immunity. The complement system has been described as a group of proteins that assist, or complement, the innate and adaptive immune systems’ antibodies in killing pathogens. The primary role of the complement system is to identify common pathogens and, through a number of proteolytic cascades, lyse the pathogen and generate an inflammatory response through production and release of proinflammatory mediators.2,3 The complement proteins, which number C1-C9, play sequential roles following antibody binding to the cell surface and result in opsonization and cell lysis. Although this is a key regulatory function for eliminating invading bacteria and pathogens, limitation of complement activation is required for prevention of lysis of the hosts’ cells, including the red blood cells, endothelial cells, and other cells in the vascular space. To prevent lysis of host cell membranes, the complement system expresses factors, such as FH, that limit activity of complement and act as a cofactor for factor I (FI) to inhibit complement on the cell surface.
FH is a key regulator that limits complement from lysing or destroying the host cell. A number of consequences have been associated in patients with mutations in FH, including atypical hemolytic uremic syndrome (aHUS).4-6 Although hemolytic uremic syndrome is characterized by thrombocytopenia, microangiopathic hemolytic anemia, and renal failure, aHUS has the added characteristic of not being associated with infection of Escherichia coli commonly found with HUS. aHUS results in damage to the microvasculature and endothelium because of rare genetic defects in FH. One of the most common vascular dysfunctions observed with aHUS is thrombotic microangiopathies (TMAs), a serious disease originally observed in children and often resulting in anemia, renal failure, and death. It is now appreciated that aHUS can present in adults as well as in children and can be treated with anticomplement therapy such as eculizumab when properly diagnosed.7 However, the underlying mechanism by which FH mutations result in TMAs is not well understood.
The focus of the article by Ueda et al is to assess the importance of a rare mutation in FH that results in impairment of its interaction with host cells and determine how this mutation identified as a W1183R (W1206R in mouse) change in human patients regulates both atypical complement on the host cell as well as that of the plasma complement activity.8 Because of the role of FH in regulating complement by acting as a cofactor for FI and interacting with the host cell membrane, it is important to determine how this mutation toward the carboxyl end of FH translates to aHUS.4,5,9 The researchers were able to show that the murine equivalent of this mutation, when expressed in human embryonic kidney cells, exhibited increased binding to heparin as well as increased levels of complement in plasma, suggesting that the amino end of FH is still able to interact in plasma and that consumption of C3 and C5 has not occurred in the mouse model. However, FH was no longer able to interact with the host cell, and cell lysis was not observed. This dissociation of the function of this FH mutation from the regulation of plasma C3 suggests that these two functions may be able to be individually regulated by targeted therapy to different regions of FH.
FH mutations in the carboxyl domains that contact the host cell have been associated previously with TMAs and renal failure. Interestingly, Song’s laboratory was able to show for the first time that this mutation was also associated with a number of large-vessel thrombotic angiopathies, including stroke, retinopathy, and multiorgan thrombosis (see figure). This was shown to be due in part to altered dilatory properties in the vessel endothelium and smooth muscle cells, and the FH mutation was further shown to cause dysregulation of the alternative pathway complement on the cell surface in the mouse. Although the FH mutation was observed in multiple vascular beds, leading to a number of pathophysiological conditions, it was not observed in fetus, suggesting that FH and complement systems may not fully develop prenatally.
This work is of exceptional importance not only for its elucidation of where in the complement system (plasma or cell membrane) the W1206R mutation is creating a dysregulated condition resulting in aHUS, but perhaps more important, this study demonstrates that mutations in FH can lead to both small-vessel pathology as observed with TMAs and renal failure, as well as large-vessel thrombosis, including retinopathy and stroke. Of note is the observation that the dysfunction is completely abrogated in mice lacking C3, supporting C3 regulation by FH as the underlying condition by which mutations in FH result in systemic thrombotic angiopathies. Finally, the work in this study will serve as a starting point for future work to begin to elucidate the role of complement and its many modifiers in regulation of vessel integrity, hemostasis, and thrombosis in both large and small vessels.10
Conflict-of-interest disclosure: The author declares no competing financial interests.