In this issue of Blood, Naik et al evaluate the link between platelet stimulation and mitogen-activated protein kinase (MAPK) activation and find a critical role for apoptosis signal-regulating kinase 1 (ASK1) in platelet function and thrombus formation.1
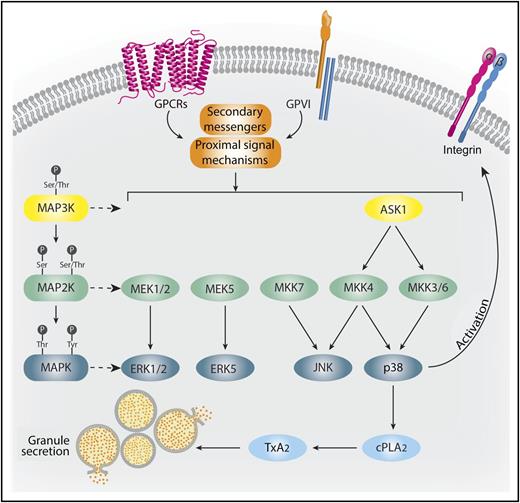
ASK1 is a critical MAP3K in platelet activation. Physiological agonists acting through either G-protein-coupled receptors (GPCRs) or glycoprotein VI (GPVI) stimulate secondary messengers and proximal signaling events that activate MAP3Ks, such as ASK1. ASK1 activates MAP2Ks, including MKKs 3, 4, and 6, leading to the stimulation of p38.10 p38 both phosphorylates cytoplasmic phospholipase A2 (cPLA2), stimulating TxA2 generation, and facilitates integrin activation and granule secretion. JNK, ERK1/2, and ERK5 are controlled by alternative upstream activation mechanisms. Professional illustration by Somersault18:24.
ASK1 is a critical MAP3K in platelet activation. Physiological agonists acting through either G-protein-coupled receptors (GPCRs) or glycoprotein VI (GPVI) stimulate secondary messengers and proximal signaling events that activate MAP3Ks, such as ASK1. ASK1 activates MAP2Ks, including MKKs 3, 4, and 6, leading to the stimulation of p38.10 p38 both phosphorylates cytoplasmic phospholipase A2 (cPLA2), stimulating TxA2 generation, and facilitates integrin activation and granule secretion. JNK, ERK1/2, and ERK5 are controlled by alternative upstream activation mechanisms. Professional illustration by Somersault18:24.
MAPKs were discovered over 30 years ago, when Sturgill and Ray2 detected activation of a serine/threonine kinase following stimulation of adipocytes by insulin. The molecular cloning of a MAPK, termed extracellular signal-regulated kinase 1 (ERK1), revealed that the complementary DNA was nearly 50% homologous to that of kinases in the yeast mating pathway.3 Purification and cloning of kinases that respond to physiological stress and are activated through concomitant phosphorylation of tyrosine and threonine residues identified 2 additional kinases, JNK4 and p38.5 Using a combination of complementation experiments in yeast and biochemical approaches in vertebrate systems, a cascade of MAPKs organized into separate but related pathways was identified. Concurrently, a vast array of MAPK substrates was identified, including transcription factors, membrane receptors, regulatory proteins, membrane trafficking proteins, survival factors, structural proteins, and many others. Thus, MAPKs represent a family of ubiquitously expressed, evolutionarily conserved kinases essential for regulating a wide variety of responses of eukaryotic cells to environmental cues.
The importance of the MAPK family in platelet activation has been appreciated for more than 2 decades. MAPKs are essential for platelet aggregation induced by several agonists and have been demonstrated to function in the activation of αIIbβ3.6 Both the ERK and p38 pathways have been invoked in this activity. Platelet MAPKs also function in pathways leading to platelet granule release, thromboxane A2 (TxA2) generation, and spreading.6-9 Consistent with their importance in platelet activation, MAPKs are essential for thrombus formation. Pharmacological inhibition of ERK2 blocks arteriolar thrombosis.7 Thrombosis is also impaired in mice with a homozygous deficiency of JNK8 or a heterozygous deficiency of p38.9 Yet, despite the proven importance of MAPK family members in platelet function, we have only a superficial understanding of how MAPK pathways are organized in the platelet.
Our current understanding of platelet MAPK kinase organization is based heavily on pathways charted in other cell types. This work arranges the MAPK family into 4 pathways: ERK1/2, ERK5, JNK, and p38. These MAPKs are activated by kinases, termed MAPK kinases (MAP2K, MKK) (see figure). ERK1/2 is phosphorylated by MEK1/2, ERK5 is phosphorylated by MEK5, JNK is phosphorylated by MKK4/MKK7, and p38 is typically phosphorylated by MKK3/MKK6, but can also be activated by MKK4. Proximal to the MKKs are another layer of kinases, termed MAPKK kinases (MAP3K, MKKK). MAP3Ks are activated by several types of secondary messengers and small G-proteins and they activate MAP2Ks via serine/threonine phosphorylation. ASK1 is a MAP3K that has previously been implicated in JNK and p38 pathways.10 It functions in a signalosome, associating with negative regulators, such as thioredoxin, 14-3-3, or calcium, and integrin binding protein 1 under resting conditions. In response to cellular stress, ASK1 disassociates from these negative regulators, which allows for homophilic interactions between ASK1 monomers enabling its activation.10 In nucleated cells, activated ASK1 can phosphorylate MKK4/MMK7-JNK and MKK3/MKK6-p38 signaling cascades. Despite a growing appreciation of the importance of ASK1 in stress responses in nucleated cells, the role of ASK1 in platelet signaling was unknown.
Naik et al have now evaluated the role of ASK1 in platelet activation and thrombus formation. In their initial studies, they showed that platelets contain ASK1 and that platelet ASK1 is phosphorylated after stimulation of platelets with several platelet agonists. They subsequently evaluated platelets from Ask1−/− mice and found that platelet aggregation in response to submaximal doses of agonists was defective. ASK1 deficiency impaired both αIIbβ3 activation and platelet granule secretion, but had no effect on agonist-induced stimulation of intracellular Ca2+ flux. Thus, the cause of the platelet function defects appeared to be downstream of increased Ca2+ flux.
In contrast to Ca2+ flux, agonist-induced TxA2 generation was markedly impaired in Ask1−/− platelets. The key enzyme involved in TxaA2 generation is cPLA2. The authors showed that agonist-induced activation of cPLA2 by phosphorylation of S505 was absent in Ask1−/− mice. To assess what MAPKs are responsible for cPLA2 phosphorylation downstream of ASK1, the authors evaluated agonist-induced phosphorylation of ERK1/2, JNK, and p38 in Ask1−/− mice. Importantly, only p38 phosphorylation was impaired, indicating that p38 is responsible for cPLA2 phosphorylation. Further evaluation showed that MKK3, MKK4, and MKK6 are all under the control of ASK1 in platelets, whereas MKK7 is not (see figure).
The authors next determined whether the functional platelet abnormalities in Ask1−/− mice were physiologically significant. An early indication of such significance was that the authors found fecal blood in 70% of Ask1−/− mice compared with 20% of wild-type mice. Tail bleeding studies confirmed that Ask1−/− mice have prolonged bleeding times. Carotid occlusion following FeCl3 exposure was significantly delayed in Ask1−/− mice compared with wild-type controls, indicating a thrombosis defect. To prove that the defect in thrombus formation was secondary to ASK1 deficiency in platelets, the authors immunodepleted platelets from both Ask1−/− mice and wild-type platelets and infused back either wild-type or ASK1-deficient platelets. Only those animals with ASK1-deficient platelets showed delayed occlusion. Consistent with the premise that platelet ASK1 is essential for normal thrombus formation, survival from pulmonary embolization induced by an infusion of collagen and epinephrine was increased in Ask1−/− mice.
The studies by Naik et al provide substantial insight into the MAP3K level (see figure) of signaling controlling MAPK pathways in platelets. These results demonstrate that ASK1 is essential for both normal hemostasis and thrombosis. Several reagents targeting MAPK pathways are currently being tested in clinical trials. The enhanced understanding of MAPK signaling pathways in platelets may help direct the evaluation of bleeding as a side effect in clinical trials using these reagents.
Conflict-of-interest disclosure: The author declares no competing financial interests.