Abstract
The discovery of a disintegrin-like and metalloproteinase with thrombospondin type 1 motif, member 13 (ADAMTS13) revolutionized our approach to thrombotic thrombocytopenic purpura (TTP). Inherited or acquired ADAMTS13 deficiency allows the unrestrained growth of microthrombi that are composed of von Willebrand factor and platelets, which account for the thrombocytopenia, hemolytic anemia, schistocytes, and tissue injury that characterize TTP. Most patients with acquired TTP respond to a combination of plasma exchange and rituximab, but some die or acquire irreversible neurological deficits before they can respond, and relapses can occur unpredictably. However, knowledge of the pathophysiology of TTP has inspired new ways to prevent early deaths by targeting autoantibody production, replenishing ADAMTS13, and blocking microvascular thrombosis despite persistent ADAMTS13 deficiency. In addition, monitoring ADAMTS13 has the potential to identify patients who are at risk of relapse in time for preventive therapy.
Introduction
Thrombotic thrombocytopenic purpura (TTP) has long been recognized as a dire hematologic emergency. Historically, nearly all patients died during the first month of illness with severe hemolytic anemia, abundant schistocytes, profound thrombocytopenia, neurological deficits, renal injury, and fever.1 Almost 40 years ago, plasma exchange was found to be an effective treatment, and by 1991, a pivotal clinical trial established plasma exchange as the standard of care, enabling ≥80% of patients with TTP to survive.2 The efficacy of plasma exchange made it feasible to treat patients earlier, before the development of potentially irreversible tissue injury. Why plasma exchange worked was unknown, but Joel Moake gained an important insight in 1982 by discovering that extremely large or “ultralarge” von Willebrand factor (VWF) multimers were associated with chronic relapsing TTP. He proposed that plasma contains a factor, missing in TTP, that regulates the size of VWF multimers and prevents microvascular thrombosis.3 During the last 20 years, we finally learned the identity of that missing factor: a metalloprotease named a disintegrin-like and metalloproteinase with thrombospondin type 1 motif, member 13 (ADAMTS13), and we are still working out the implications of this knowledge. This review will discuss recent advances in understanding the relationship between the pathophysiology of TTP and its signs, symptoms, diagnosis, and management.
VWF-dependent platelet adhesion and TTP
VWF multimers are composed of identical subunits that are linked together by disulfide bonds (Figure 1). Each subunit consists of several kinds of repeated structural domains, and has binding sites for components of connective tissue, such as collagen, as well as platelet membrane glycoproteins, factor VIII, and ADAMTS13.
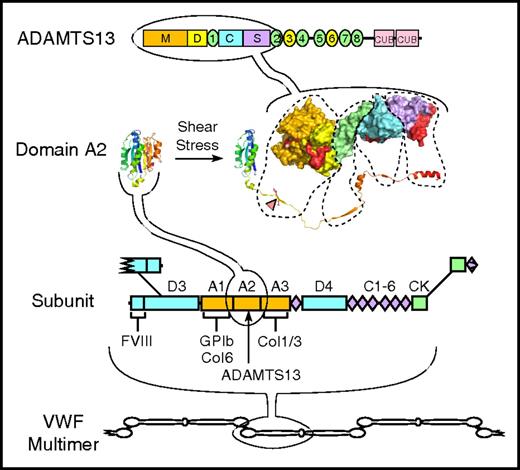
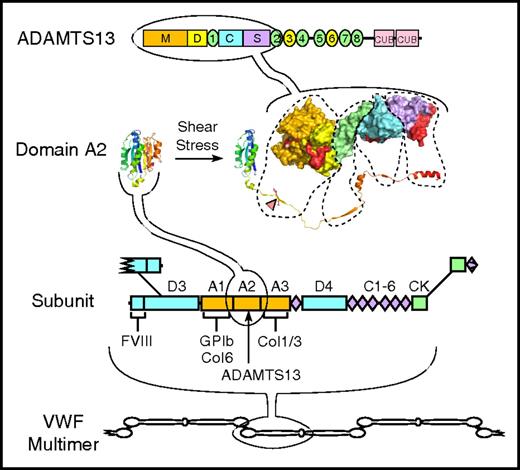
Structure of VWF and ADAMTS13. VWF multimers may contain ≥40 ∼250 kDa subunits linked together by disulfide bonds between C-terminal cystine-knot (CK) domains and N-terminal D3 domains. The VWF subunit is composed mostly of repeated A, C, and D domains and has binding sites for many proteins, including factor VIII, platelet GPIb, and collagens (Col) 1, 3, and 6. The A2 domain has a globular structure in native VWF, but unfolds in response to fluid shear stress to expose a Tyr-Met bond (red triangle) that is cleaved by ADAMTS13. ADAMTS13 is a multidomain protein with metalloprotease (M), disintegrin-like (D), thrombospondin type 1, Cys-rich, spacer, 7 more thrombospondin type 1 repeats, and 2 CUB domains. A molecular model of the proximal domains shows sites (shaded red) that interact with the extended sequence of the A2 domain.
Structure of VWF and ADAMTS13. VWF multimers may contain ≥40 ∼250 kDa subunits linked together by disulfide bonds between C-terminal cystine-knot (CK) domains and N-terminal D3 domains. The VWF subunit is composed mostly of repeated A, C, and D domains and has binding sites for many proteins, including factor VIII, platelet GPIb, and collagens (Col) 1, 3, and 6. The A2 domain has a globular structure in native VWF, but unfolds in response to fluid shear stress to expose a Tyr-Met bond (red triangle) that is cleaved by ADAMTS13. ADAMTS13 is a multidomain protein with metalloprotease (M), disintegrin-like (D), thrombospondin type 1, Cys-rich, spacer, 7 more thrombospondin type 1 repeats, and 2 CUB domains. A molecular model of the proximal domains shows sites (shaded red) that interact with the extended sequence of the A2 domain.
VWF is secreted from endothelial cells as ultralarge multimers, and ADAMTS13 progressively reduces the size of these VWF multimers as they circulate in the blood. Under low-shear conditions, VWF multimers adopt a loosely coiled, condensed shape4 as a result of weak interactions between VWF monomers.5,6 Above a critical shear rate, VWF multimers extend in the direction of elongational flow4 and experience tensile force. The longer the multimer, the greater the force, which can be sufficient to unfold the VWF A2 domain and expose a cryptic Tyr-Met bond that ADAMTS13 recognizes and cleaves.7
VWF multimers can bind platelets on the endothelial cell surface or at sites of vascular injury (Figure 2).8 Compared with VWF in solution, tethered VWF requires much lower shear stress to induce conformational changes that promote binding to platelets or cleavage by ADAMTS13. In addition, platelets bound to VWF present a large surface to flowing blood and transmit correspondingly larger forces to VWF than can be achieved without platelets. The increased tensile force accelerates the unfolding and cleavage of VWF A2 domains by ADAMTS13,9,10 releasing smaller VWF multimers along with any attached platelets.
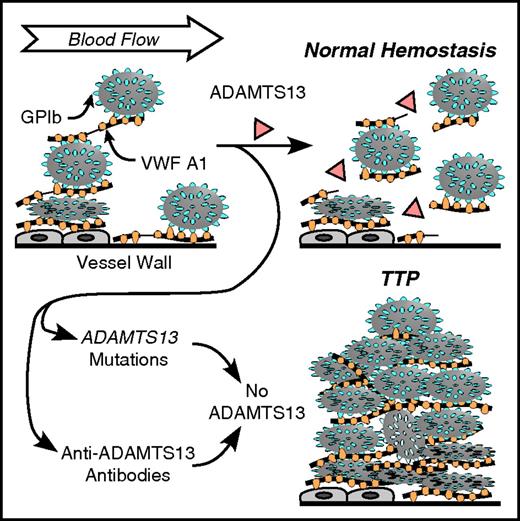
Role of VWF and ADAMTS13 in platelet adhesion. VWF multimers may adhere to endothelial cells or to connective tissue in the vessel wall. Platelet GPIb binds to the VWF A1 domain. Flowing blood applies force to the platelets that stretches VWF and exposes a cleavage site for ADAMTS13 in the A2 domain (thin lines in VWF multimers attacked by ADAMTS13). Cleavage of VWF limits the growth of intravascular thrombi. Congenital or acquired ADAMTS13 deficiency allows excessive platelet deposition, causing microvascular thrombosis and TTP.
Role of VWF and ADAMTS13 in platelet adhesion. VWF multimers may adhere to endothelial cells or to connective tissue in the vessel wall. Platelet GPIb binds to the VWF A1 domain. Flowing blood applies force to the platelets that stretches VWF and exposes a cleavage site for ADAMTS13 in the A2 domain (thin lines in VWF multimers attacked by ADAMTS13). Cleavage of VWF limits the growth of intravascular thrombi. Congenital or acquired ADAMTS13 deficiency allows excessive platelet deposition, causing microvascular thrombosis and TTP.
Force-induced proteolytic cleavage of VWF limits thrombus growth and prevents the microvascular thrombosis, tissue ischemia, and infarction that are characteristic of TTP. This model provides a framework for understanding the pathophysiology of TTP.
Consequences of ADAMTS13 deficiency: symptoms and signs of TTP
The life cycle of VWF is quite different in the absence of ADAMTS13 (Figure 2). Ultralarge VWF multimers that are secreted by endothelial cells bind tightly to platelets even at low-shear forces,11 forming aggregates that can embolize and occlude downstream arterioles. Platelet-VWF aggregates may also form directly in these resistance vessels, where high-fluid shear stress can induce VWF to bind platelets.
An adult has only 10 to 20 mL of circulating platelets, which can easily be sequestered in VWF-platelet aggregates to cause profound thrombocytopenia. For example, administering desmopressin to a patient with von Willebrand disease type 2B causes the acute release of hyperadhesive VWF that binds to platelets, which then disappear from the blood for a few hours until ADAMTS13 cleaves the VWF and returns the platelets to the circulation.12 The microvascular thrombi in TTP consist of platelets and VWF, with very little fibrin, which is consistent with the deposition of mainly unactivated platelets that do not provoke blood clotting and fibrin deposition. This pathology differs from the fibrin-rich, platelet-poor thrombi that occur in Shiga toxin–associated hemolytic uremic syndrome and atypical hemolytic uremic syndrome, reflecting distinct mechanisms of disease.13-15
Thrombi in TTP occur in all tissues, but are rare in the lungs and liver, which may be spared because blood circulates through these organs at low pressure with low-shear forces that are insufficient to promote VWF platelet binding. Lesions occur at high density in the heart, pancreas, kidney, and brain. Nevertheless, these tissues exhibit relatively little necrosis14 when compared with the extensive damage caused by the injection of rigid microspheres into the coronary or carotid circulation of animals.16,17 This disparity suggests that lesions in TTP may not always persist long enough to cause widespread necrosis. Transient occlusion would be consistent with the common finding that neurological signs in TTP can be fleeting.
Alternatively, thrombi in TTP may be partially occlusive and allow red blood cells to pass around or through them.18 Red blood cells in partially occluded resistance vessels would experience high-shear forces, which could explain the occurrence of hemolytic anemia with schistocytes. A similar mechanism is thought to cause thrombotic microangiopathy in malignant hypertension.19
Recent studies suggest that VWF may contribute more directly to the production of schistocytes in TTP. Fluid shear stress causes VWF multimers to associate into thick fibers, and ADAMTS13 inhibits this process.20,21 In engineered in vitro microvessels with diameters <300 µm, like small arteries and arterioles, VWF fibers can repeatedly cross the lumen and extend for up to several millimeters. The fibers obstruct blood flow, forcing red blood cells to fold over them and tear into fragments,21 suggesting that VWF fibers in vivo may produce schistocytes in TTP.
Thus, ADAMTS13 deficiency can cause the cardinal features of TTP, thrombocytopenia and microangiopathic hemolytic anemia, by failing to regulate VWF-dependent platelet adhesion. The centrality of this mechanism is clearly illustrated by the results of administering an inhibitory anti-ADAMTS13 antibody to baboons. Without additional inciting triggers, the animals developed severe thrombocytopenia, microangiopathic hemolytic anemia, elevated lactate dehydrogenase, and characteristic VWF- and platelet-rich lesions in the heart, brain, kidney, and spleen. Furthermore, blocking the interaction between VWF and platelet glycoprotein Ib (GPIb) prevented the development of acute TTP in this model.15,22
In principle, the same pathology might be produced by VWF mutations that prevent the cleavage of the target Tyr-Met bond in the A2 domain of the VWF subunit, or by autoantibodies against VWF that block access by ADAMTS13. No such mutation or antibody has been observed in humans, but an elegant mouse model confirms that uncleavable VWF can cause TTP. Morioka and colleagues23 designed a mouse VWF variant with an engineered disulfide bond in the VWF A2 domain that prevents unfolding under shear stress and blocks cleavage by ADAMTS13. When this VWF was expressed in mice with normal levels of ADAMTS13, the mice developed thrombocytopenia, hemolysis with schistocytes, renal dysfunction, and splenomegaly. Thrombotic microangiopathy also occurs in Adamts13−/− mice after the infusion of human VWF.24 Human VWF is relatively resistant to cleavage by mouse ADAMTS13, and high doses of human VWF induce thrombocytopenia and myocardial thrombosis even in mice that have functional ADAMTS13.25 The similar phenotypes of mouse models with ADAMTS13 deficiency and uncleavable VWF indicate that VWF is the major physiologically relevant substrate of ADAMTS13.
Asynchronous development of TTP signs and symptoms
TTP is commonly diagnosed in patients with hemolytic anemia, schistocytes, thrombocytopenia, relatively preserved renal function, and no other cause for these findings, such as malignant hypertension, sepsis, or cancer.2 Most such patients have severe ADAMTS13 deficiency, whether or not they have signs of tissue injury.26 However, occasional patients with ADAMTS13 <10% of normal (<10 IU/dL) present with life-threatening microvascular thrombosis, including stroke or seizures, long before they develop microangiopathic hemolytic anemia or thrombocytopenia.27-31
This variability is consistent with the pathophysiology of TTP. Thrombocytopenia is a consequence of platelet sequestration in the microvasculature, which need not occur in a high-shear location. Hemolysis and the production of schistocytes require obstruction of blood flow in high-shear regions by microthrombi, or perhaps by VWF fibers, which need not be associated with thrombocytopenia. Tissue injury requires ischemia, which depends on the location of a thrombus and the quality of collateral circulation. A small thrombus in a critical place can cause stroke or cardiac injury without hemolysis or thrombocytopenia. In short, we should not be surprised by the asynchronous appearance and disappearance of signs and symptoms in TTP. Conversely, even the most classic presentation is not specific for TTP. An important clinical corollary is that the diagnosis of TTP, especially of atypical presentations, depends on ADAMTS13 testing.
Asymptomatic ADAMTS13 deficiency and the risk of relapsing TTP
Longitudinal ADAMTS13 testing in TTP has established persistent or recurrent ADAMTS13 deficiency as a strong risk factor for relapse during the following months.32-36 However, some patients respond to treatment with a complete hematological response despite persistence of ADAMTS13 <10%, and some patients with chronic ADAMTS13 <10% remain asymptomatic for long periods, sometimes years.36-38 For example, among 67 patients with acquired TTP, 20 had relapses associated with ADAMTS13 deficiency after a median interval of 2 years. Interestingly, 8 patients had ≥1 ADAMTS13 value <10% that was not followed by a relapse for >1 year, and 3 of them were asymptomatic during 10 years of sustained severe ADAMTS13 deficiency.36
This phenomenon is also well documented for inherited ADAMTS13 deficiency. Many patients begin to have hemolysis and thrombocytopenia as infants, but approximately one-half of them appear healthy until adulthood.39 In particular, women with inherited ADAMTS13 deficiency often have their first recognized episode of TTP during their first pregnancy.40,41
Triggers and modifiers of TTP susceptibility
The demographics and variable course of ADAMTS13 deficiency suggests that other factors in the host or the environment influence the risk of developing active thrombotic microangiopathy. Several have been proposed, with varying degrees of supporting evidence.
Inherited risk factors
As also observed for systemic lupus erythematosus (SLE), the likelihood of developing TTP is increased substantially for women of African ancestry, with the highest incidence between 30 and 50 years of age. In fact, some patients with SLE later develop TTP, and some patients with TTP have additional abnormalities that occur in SLE and other autoimmune disorders.42,43 These similarities suggest a common genetic predisposition for SLE and TTP that may be triggered by the hormonal environment in women of childbearing age. HLA-DRB1*11 is another risk factor, possibly because dendritic cells bearing HLA-DRB1*11 can present peptides derived from the CUB2 domain of ADAMTS13 to activate ADAMTS13-specific self-reactive CD4+ T cells and thereby enhance the production of autoantibodies by ADAMTS13-specific B cells.44 Although preliminary, these findings suggest that understanding the immune dysregulation in TTP could lead to the identification of patients at high risk and perhaps to the development of treatments to restore antigen-specific tolerance.
VWF level
VWF is required to cause TTP in Adamts13−/−-deficient mice, but varying the level of VWF between 20 and 120 U/mL does not appear to affect the occurrence or severity of disease, suggesting that a threshold level of VWF is sufficient, but more confers little additional risk.45,46 However, humans appear more sensitive than mice to changes in VWF. For example, the administration of desmopressin to a few patients with acquired47 or inherited ADAMTS13 deficiency48,49 promptly caused acute hemolysis and thrombocytopenia, presumably secondary to the secretion of ultralarge VWF multimers. Women with inherited ADAMTS13 deficiency reliably develop TTP during pregnancy,40,41,50 and the pregnancy-associated increase in VWF level is a plausible contributing factor. Thus, changes in VWF secretion, multimer distribution, and plasma level may trigger TTP.
ADAMTS13 activity
For inherited ADAMTS13 deficiency, the risk of developing active disease appears to depend inversely on the level of residual ADAMTS13 activity below the threshold of 5% to 10% that protects against thrombotic microangiopathy. ADAMTS13 activity <1% to 2% has been associated with first presentation during childhood with relapsing disease and with a need for prophylactic plasma infusions.51 Mutations in patients with childhood onset or relapsing TTP also were associated with acute renal impairment during episodes of disease.52 Additional information about genotype and phenotype correlations should be forthcoming from a multi-institutional registry of patients with inherited TTP (identifier NCT01257269, clinicaltrials.gov).
Whether residual ADAMTS13 activity influences the risk of relapse in acquired TTP has not been reported. However, the most common clinical laboratory assays for ADAMTS13 have a limit of detection of 5%,53 and more sensitive methods are needed to address this question.
Inflammation
In addition to pregnancy,40,41,50 common triggers of TTP in acquired or inherited ADAMTS13 deficiency include infections and surgery.54-56 These inflammatory conditions may promote microvascular thrombosis through endothelial injury or activation that induces the secretion or promotes the adhesion of VWF.
Susceptibility to infectious stress also may depend on toll-like receptor 9 (TLR-9), which recognizes bacterial and viral DNA and induces a variety of immune effector cells to produce inflammatory cytokines. In a recent study, functionally relevant polymorphisms of TLR-9 were more common in patients with TTP compared with controls.56
Inflammation is associated with oxidative stress, which has the potential to enhance platelet adhesion by modifying either ADAMTS13 or VWF. For example, hypochlorous acid, a product of neutrophil myeloperoxidase, can inactivate ADAMTS1357 and render VWF resistant to proteolysis.58,59 At present, the available data do not conclusively show this mechanism contributes to the pathophysiology of TTP.
Other proteases
Once the VWF A2 domain is unfolded, it can be cleaved in vitro by serine proteases, such as thrombin, plasmin, and the leukocyte proteases elastase, cathepsin G, proteinase 3, and granzyme B.60-63 One or more of these proteases might compensate at least partly for ADAMTS13 deficiency and protect against thrombotic microangiopathy, if they could avoid rapid inhibition by plasma α1-proteinase inhibitor, antithrombin, α2-antiplasmin, or α2-macroglobulin.
To date, plasmin is the strongest candidate for significant activity toward VWF in vivo. Plasminogen frequently is activated during acute episodes of TTP, as shown by increased plasma levels of plasmin-α2-antiplasmin complexes,64 and plasmin cleaves VWF multimers in a shear-dependent manner.63,65 When TTP was induced in Adamts−/− mice by infusion of human VWF, the administration of streptokinase a few minutes later prevented the development of thrombocytopenia.64 In contrast, plasmin can cleave and inactivate ADAMTS13, and a hyperfibrinolytic state in a patient with preexisting mild ADAMTS13 deficiency has been proposed to cause TTP by degrading ADAMTS13.66 Whether plasminogen activation can exacerbate or attenuate the risk of TTP in humans remains unknown.
High-density lipoprotein
Under shear stress, VWF multimers interact noncovalently to form long prothrombotic fibers that bind platelets avidly. Chung and colleagues recently discovered that physiological levels of plasma high-density lipoprotein (HDL) or ApoA-I inhibit shear-induced VWF self-association and thereby inhibit platelet adhesion. Also, in a mouse model of TTP, HDL largely prevented thrombocytopenia induced by infusing human VWF into Adamts13−/− mice.67 These results suggest that HDL levels could influence the risk of active TTP in humans with ADAMTS13 deficiency.
Pathophysiology as a guide to the treatment of TTP
Plasma infusions are an effective treatment for inherited TTP, but are inconvenient and often induce allergic reactions. Plasma exchange remains the standard treatment of acquired TTP. Most patients respond to a combination of plasma exchange and rituximab, but some patients die or acquire irreversible neurological deficits before they can respond, and relapses can occur unpredictably. However, knowledge of the pathophysiology of TTP has inspired new ways to address these problems (Figure 3).
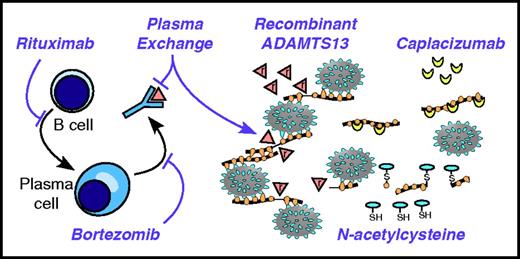
Targeting the pathophysiology of TTP. Anti-CD20 agents, such as rituximab, kill B cells and prevent their differentiation into plasma cells. Long-lived plasma cells may be targeted by agents with activity against multiple myeloma, such as bortezomib. Anti-ADAMTS13 autoantibodies are removed by plasma exchange. ADAMTS13 can be replaced by plasma exchange and potentially by recombinant ADAMTS13 (indicated by triangles marked with “r”). Large thrombogenic VWF multimers can be shortened by reducing agents like N-acetylcysteine. The thiol (SH) of N-acetylcysteine attacks and cleaves disulfide bonds of VWF, making smaller VWF multimers disulfide-linked (S) to the cysteine moiety. Inhibitors of VWF-platelet interactions, such as caplacizumab, can stop the progression of TTP in the absence of ADAMTS13.
Targeting the pathophysiology of TTP. Anti-CD20 agents, such as rituximab, kill B cells and prevent their differentiation into plasma cells. Long-lived plasma cells may be targeted by agents with activity against multiple myeloma, such as bortezomib. Anti-ADAMTS13 autoantibodies are removed by plasma exchange. ADAMTS13 can be replaced by plasma exchange and potentially by recombinant ADAMTS13 (indicated by triangles marked with “r”). Large thrombogenic VWF multimers can be shortened by reducing agents like N-acetylcysteine. The thiol (SH) of N-acetylcysteine attacks and cleaves disulfide bonds of VWF, making smaller VWF multimers disulfide-linked (S) to the cysteine moiety. Inhibitors of VWF-platelet interactions, such as caplacizumab, can stop the progression of TTP in the absence of ADAMTS13.
Replacing ADAMTS13
Purified ADAMTS13 would be ideal for the treatment of inherited ADAMTS13 deficiency. It would prevent infusion reactions caused by sensitization to plasma proteins, make home infusions feasible, and simplify ADAMTS13 replacement therapy during pregnancy. Recombinant human ADAMTS13 is under development for this purpose. In a first-in-human phase 1 trial in inherited ADAMTS13 deficiency (identifier NCT02216084, clinicaltrials.gov), recombinant ADAMTS13 was safe and well tolerated, with pharmacokinetics similar to ADAMTS13 in plasma.68
Recombinant ADAMTS13 may also be useful in acquired TTP because inhibitor levels frequently are low enough to overcome with exogenous ADAMTS13 in vitro.69 Recombinant ADAMTS13 also was shown to prevent thrombocytopenia, hemolysis, and microvascular thrombosis in a rat model of TTP induced by infusing polyclonal anti-ADAMTS13 antibodies and human VWF.70 These results suggest that recombinant ADAMTS13 might be effective in combination with or as a substitute for plasma exchange in patients with acquired TTP and low titer inhibitors.
Almost all patients with acquired TTP have IgG autoantibodies that recognize the same epitope in the spacer domain of ADAMTS13. Mutations in this epitope have been identified that prevent recognition by most autoantibodies while preserving ADAMTS13 function, suggesting that inhibitor-resistant ADAMTS13 might be therapeutically useful.71 The efficacy of this strategy could be reduced by the presence of autoantibodies against other ADAMTS13 domains and by immune responses to the modified ADAMTS13. In another approach to circumvent inhibitors, human ADAMTS13 was expressed and stored within mouse platelets to make it inaccessible to inhibitors. Mice with these transgenic platelets were resistant to thrombocytopenia, microvascular thrombosis, and death in a model of TTP, despite the presence of high-titer anti-ADAMTS13 antibodies.72 In principle, these strategies could be effective for acquired TTP regardless of inhibitor titer.
Suppressing anti-ADAMTS13 antibodies
Plasma exchange and glucocorticoids can carry most patients through an acute episode of acquired TTP, but relapses are common because these treatments do not reliably eliminate the cells that make anti-ADAMTS13 antibodies. However, rituximab is remarkably effective at killing the ADAMTS13-specific B cells that mature into short-lived plasma cells and stopping autoantibody production. Whether started immediately or reserved for patients refractory to plasma exchange, rituximab shortens the time to response and reduces the incidence of relapse.73-77 Rituximab usually is given as 4 weekly doses of 375 mg/m2, but 3 doses at shorter intervals may be as effective and more convenient (identifier NCT00907751, clinicaltrials.gov).74,78 The optimal dose of rituximab in TTP is not known, and monitoring B-cell depletion may be useful to assure an adequate therapeutic effect.78 Patients have responded to doses as low as 100 mg/m2, and a pilot study of low-dose rituximab combined with plasma exchange in acquired TTP is in progress (NCT01554514).79,80 A clinical trial to compare low-dose and standard-dose regimens would be useful.
Occasional patients are refractory to rituximab at presentation or become so eventually, probably because they harbor long-lived plasma cells that make anti-ADAMTS13 antibodies. Plasma cells are resistant to conventional immunosuppression, but may be susceptible to treatments developed for multiple myeloma. For example, bortezomib has been used as salvage therapy for ≥12 patients with TTP, of whom many were reported to respond.81-83 It seems likely that other agents developed for B-cell malignancies or multiple myeloma will be tested in autoimmune disorders, and some may find a place in the treatment of refractory TTP.
Depolymerizing VWF multimers
The intersubunit disulfide bonds of VWF multimers are sensitive to reducing agents, which suggests that the reduction of VWF could be therapeutically useful. In preclinical testing, the reducing agent N-acetylcysteine depolymerized VWF multimers and protected Adamts13−/− mice from thrombosis of mesenteric venules.84 N-acetylcysteine also reduced a key disulfide bond in the A1 domain, which could impair binding to platelet GPIb.84 In a model of TTP induced by injecting human VWF into Adamts13−/− mice, N-acetylcysteine given immediately prevented thrombocytopenia, anemia, and myocardial necrosis; however, administration of N-acetylcysteine 1 hour after VWF infusion was not effective.85 N-acetylcysteine also did not reverse thrombocytopenia or hemolysis in a baboon model of acquired TTP, although it did decrease the level of high–molecular weight VWF multimers. Thus, N-acetylcysteine may be able to prevent VWF-dependent microvascular thrombosis, but not to dissolve preexisting thrombi.85
The use of N-acetylcysteine has been reported for ≥8 patients with TTP, of whom 3 appeared unresponsive and 5 improved. These reports did not describe the effect of N-acetylcysteine on VWF multimer size in vivo. Currently, a pilot study (identifier NCT01808521, clinicaltrials.gov) is enrolling patients with acute TTP for treatment with IV N-acetylcysteine at 150 mg/kg over 1 hour followed by 150 mg/kg over 17 hours after plasma exchange, which is similar to the doses used for acetaminophen toxicity.
The ability of ApoA1 to prevent thrombocytopenia in a mouse model of TTP suggests that targeting the self-association of VWF might be another way to reduce the effective size of VWF multimers and interrupt microvascular thrombosis in TTP.67
Inhibiting VWF-platelet interactions
Several compounds that block VWF-platelet interactions could be considered for the treatment of TTP. These include aurintricarboxylic acid,86 antibodies,22,87 DNA88 and RNA89 aptamers, and high-affinity derivatives of GPIbα90 that target VWF, as well as antibodies,91 VWF derivatives,92 and snake venom lectins93 that target GPIb. One of these compounds, a llama antibody derivative, is the subject of ongoing clinical trials.
Llamas and related species produce some antibodies that consist of only a heavy chain, which can be selected in vitro to bind specific antigens. Caplacizumab is a bivalent humanized llama immunoglobulin variable region that recognizes the human VWF A1 domain and prevents binding to platelet GPIb.94 In a phase 2, randomized study (TITAN, identifier NCT01151423, clinicaltrials.gov), 39 patients were assigned to receive placebo and 36 to receive subcutaneous caplacizumab (10 mg/day) during plasma exchange and for 30 days afterward. The time to normalization of the platelet count was significantly shorter for caplacizumab (3.0 days) vs placebo (4.6 days). Mild to moderate bleeding occurred more often with caplacizumab (54%) than placebo (38%). Serious bleeding was rare, affecting 2 patients in each study group. Patients with persistent ADAMTS13 deficiency relapsed quickly when caplacizumab was stopped, suggesting that ADAMTS13 monitoring could be useful to guide the discontinuation of therapy after the ADAMTS13 level has increased.95 Therefore, the phase 3 HERCULES study is evaluating the extended use of caplacizumab for an additional 4 weeks if the ADAMTS13 level is <10% after 30 days of treatment (identifier NCT02553317, clinicaltrials.gov). If safe and effective, early administration of caplacizumab may reduce ischemic tissue injury and decrease or even eliminate the use of plasma exchange.
Epidemiologic and experimental data implicate VWF-dependent platelet adhesion in stroke, myocardial infarction, and other conditions. Consequently, validation of VWF-GPIb interactions as a therapeutic target in TTP could encourage testing in these additional settings.
Predicting and preventing relapses
A major challenge for patients with acquired TTP is the persistent risk of an unpredictable, life-threatening relapse. Most relapses occur during the first year or 2, but relapses can occur as late as 10 or 20 years after an episode of TTP.55 Relapses are always associated with recurrent or persistent severe ADAMTS13 deficiency, which suggests that monitoring ADAMTS13 during remission might detect a decrease in ADAMTS13 in time for preemptive treatment.
The occurrence of asymptomatic ADAMTS13 deficiency indicates that the decision to treat an asymptomatic patient needs careful thought. For acquired TTP, immediate treatment with rituximab and plasma exchange prevents or delays the recurrence of ADAMTS13 deficiency and thereby reduces the risk of relapse.73,74,76,77 Administering rituximab to asymptomatic patients with acquired severe ADAMTS13 deficiency also appears to reduce the risk of relapse.75 Preventing such relapses avoids the associated hospitalization and risk of death or chronic neurocognitive disability. However, some asymptomatic patients will not relapse, and ritixumab offers them no benefit, but puts them at risk of rare complications, such as infusion reactions, hepatitis B reactivation, pulmonary fibrosis, and progressive multifocal leukoencephalopathy.
Limited data are available to compare these risks. For example, once ADAMTS13 deficiency recurs, ∼50% of patients relapse during the next 2 years,36 and preemptive treatment with rituximab prevents at least ∼50% of such relapses.75 Thus, the number needed to treat to prevent 1 relapse may be as low as ∼4. Conversely, the risk of the most serious complication of rituximab, progressive multifocal leukoencephalopathy, is estimated to be 1 per 25 00096 or 1 per 500 000 for patients with autoimmune diseases who do not have HIV or cancer,97 implying a number needed to harm of at least ∼25 000 for this adverse event. These rough estimates suggest that prophylactic treatment with rituximab may be cost effective, even after including the risk of other adverse events, but a definitive answer will require appropriate clinical trials.
Acknowledgments
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grants R01 HL72917, R01 HL130446, and U54 HL112303.
Authorship
Contribution: J.E.S. wrote the manuscript.
Conflict-of-interest disclosure: J.E.S. is a consultant for Ablynx, BioMarin, 23andMe, and Genentech.
Correspondence: J. Evan Sadler, Department of Medicine, Washington University School of Medicine, 660 S. Euclid Ave, Box 8125, St. Louis, MO 63110; e-mail: esadler@wustl.edu.