Key Points
We identified 3 novel loss-of-function variants in NUDT15 linked to thiopurine intolerance.
Our findings extended the importance of NUDT15 variation in thiopurine pharmacogenetics in diverse populations.
Abstract
Prolonged exposure to thiopurines (eg, mercaptopurine [MP]) is essential for curative therapy in acute lymphoblastic leukemia (ALL), but is also associated with frequent dose-limiting hematopoietic toxicities, which is partly explained by inherited genetic polymorphisms in drug metabolizing enzymes (eg, TPMT). Recently, our group and others identified germ line genetic variants in NUDT15 as another major cause of thiopurine-related myelosuppression, particularly in Asian and Hispanic people. In this article, we describe 3 novel NUDT15 coding variants (p.R34T, p.K35E, and p.G17_V18del) in 5 children with ALL enrolled in frontline protocols in Singapore, Taiwan, and at St. Jude Children’s Research Hospital. Patients carrying these variants experienced significant toxicity and reduced tolerance to MP across treatment protocols. Functionally, all 3 variants led to partial to complete loss of NUDT15 nucleotide diphosphatase activity and negatively influenced protein stability. In particular, the p.G17_V18del variant protein showed extremely low thermostability and was completely void of catalytic activity, thus likely to confer a high risk of thiopurine intolerance. This in-frame deletion was only seen in African and European patients, and is the first NUDT15 risk variant identified in non-Asian, non-Hispanic populations. In conclusion, we discovered 3 novel loss-of-function variants in NUDT15 associated with MP toxicity, enabling more comprehensive pharmacogenetics-based thiopurine dose adjustments across diverse populations.
Introduction
Thiopurines (eg, mercaptopurine [MP]) are critical components of treatment regimens of acute lymphoblastic leukemia (ALL) in children and adults.1-4 Although efficacious as antileukemic agents, thiopurines also have narrow therapeutic indices with common dose-limiting hematopoietic toxicity. In addition to polymorphisms in the TPMT gene,5,6 we recently identified NUDT15 as a novel thiopurine metabolizing enzyme, genetic variation of which strongly influences MP tolerance in children with ALL7,8 and patients with inflammatory bowel diseases.9 As a nucleotide diphosphatase, NUDT15 negatively affects MP cytotoxicity by converting the thioguanine triphosphate (TGTP) to thioguanine monophosphate (TGMP), reducing DNA incorporation of thioguanine, and thus attenuating DNA damage–induced apoptosis.8,10 Thus far, 4 NUDT15 variants have been associated with loss of diphosphatase activity, excessive MP activation, and myelosuppression in patients.8 Interestingly, these hypomorphic variants are largely restricted to Asian people or Asian-related ancestral groups (Native Americans),7,8 suggesting their potential origin in Asia. NUDT15-guided thiopurines dosing was considered of limited relevance in European or African patients for which NUDT15 risk variants had hitherto been rarely reported. However, a substantial proportion of patients do not have the currently known risk variants in TPMT or NUDT15, yet experience severe MP toxicity,7-9 pointing to novel variants in these or other genes that are still to be discovered. In this article, we report 3 novel NUDT15 loss-of-function variants that were associated with MP intolerance in children with ALL from diverse ancestry.
Methods
Study subjects were children treated on frontline ALL clinical trials at National University Singapore, Singapore,11 National Taiwan University Hospital, Taiwan,12 or St. Jude Children’s Research Hospital, Memphis, TN13,14 . This study was approved by the Institutional Review Board at St. Jude Children’s Research Hospital and participating institutions of respective frontline ALL protocols.
MP tolerance was defined as the average stable MP dosage (milligram per square meter per day) over >4 weeks during maintenance therapy of the Malaysia-Singapore (MaSpore) 2003 and St. Jude Total Therapy Study (TOT) XVI low-risk protocols.11,14 TOT XIIIB high-risk maintenance therapy consisted of weekly rotation of drug pairs during which MP was given 1 week for every 4 weeks at 75 mg/m2 per day.13 The patient in Taiwan did not complete maintenance therapy because of relapse, and his MP tolerance was defined on the basis of other therapy phases of continuous MP treatment. The MP dose was clinically titrated to a target white blood cell count in each clinical protocol.
Details of NUDT15 sequencing, variant functional characterization, and thiopurine metabolite assay are provided in supplemental Methods, available on the Blood Web site.
Results and discussion
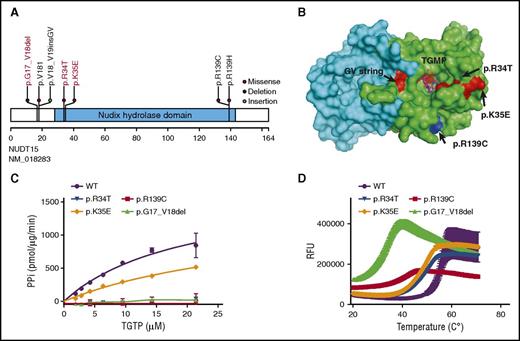
In total, we identified 3 novel coding variants in the NUDT15 gene: p.R34T, p.K35E, and p.G17_V18del, in 5 children with ALL of Asian, African, or European descent (Figure 1A-B; Table 1). The p.R34T missense variant (c.101G>C, resulting in an arginine-to-threonine conversion at the amino acid residue 34) was recurrent in 2 patients of East Asian ancestry enrolled in the MaSpore 2003 ALL protocol in Singapore and the TPOG-2002 infantile ALL protocol in Taiwan, respectively. Both patients tolerated very low doses of MP (17.9 and 16.4 mg/m2 per day, respectively) relative to that observed previously in Asian children who were heterozygous at the common risk variant p.R139C (33.1 ± 11.5 mg/m2 per day).8 The p.K35E variant substitutes the lysine residue 35 with glutamic acid and was observed in a single East Asian patient in Singapore who also had the known p.V18_V19insGV and p.R139C variants. Subsequent haplotyping confirmed that he was compound heterozygous for NUDT15 risk alleles (ie, 1 allele with the p.V18_V19insGV and p.R139C variants and the other allele with the p.K35E variant). He was exquisitely sensitive to MP with a tolerated dosage of 8.5 mg/m2 per day, similar to those homozygous at the p.R139C variant (4.3 ± 2.4 mg/m2 per day).7,8 Finally, the in-frame deletion variant, p.G17_V18del, was observed in 2 patients at St. Jude, both of whom were non-Asian (ie, of European or African descent, respectively). The patient on the TOT XVI protocol tolerated 43.5 mg/m2 per day of MP during the maintenance phase, which was comparable to patients with the heterozygous p.R139C variant, as we reported previously (47.3 ± 19.1 mg/m2 per day).7,8 The second patient with this deletion variant was treated on the TOT XIIIB protocol, for which maintenance therapy consisted of drug pairs administered in weekly rotation. Thus, for this patient, MP was given for only 1 week of every 4-week period.13 Likely because of the short duration of her MP exposure, this patient did not experience significant toxicity, and her tolerated dosage was 82.5 mg/m2 per day for that week. This is consistent with our experience with TPMT-deficient patients who also tolerated full MP dosages for short durations.15 The actual MP tolerance for this patient could have been different if she had been challenged with sustained MP dosing.
Functional characterization of 3 novel NUDT15 risk variants. (A) Shown are the positions of novel (red text) and known risk variants (black text) in the coding region of the NUDT15 gene. (B) Shown is the spatial distribution of amino acid residues affected by risk variants in the human NUDT15 protein. Presented as a homodimer, chains A and B are discriminated by color (green and cyan, respectively). Each risk variant is identified by letters as well as colors, and TGMP is shown in stick-ball presentation. The 3-dimensional structure was drawn by using PyMOL software (accession code 5LPG of the Protein Data Bank, http://www.rcsb.org/pdb). (C) NUDT15 nucleotide diphosphatase activity was determined by using the PiPer Pyrophosphate Assay (Life Technologies) for each of the 3 novel variants, with wild-type (WT) and p.R139C proteins included as controls. (D) NUDT15 protein thermostability was measured by using SYPRO Orange (Molecular Probes) for each of the 3 variants, with wild-type and the p.R139C variant included as controls. The inflection point of each curve indicates the temperature for protein unfolding and is thus a measurement of stability. RFU, relative fluorescence unit.
Functional characterization of 3 novel NUDT15 risk variants. (A) Shown are the positions of novel (red text) and known risk variants (black text) in the coding region of the NUDT15 gene. (B) Shown is the spatial distribution of amino acid residues affected by risk variants in the human NUDT15 protein. Presented as a homodimer, chains A and B are discriminated by color (green and cyan, respectively). Each risk variant is identified by letters as well as colors, and TGMP is shown in stick-ball presentation. The 3-dimensional structure was drawn by using PyMOL software (accession code 5LPG of the Protein Data Bank, http://www.rcsb.org/pdb). (C) NUDT15 nucleotide diphosphatase activity was determined by using the PiPer Pyrophosphate Assay (Life Technologies) for each of the 3 novel variants, with wild-type (WT) and p.R139C proteins included as controls. (D) NUDT15 protein thermostability was measured by using SYPRO Orange (Molecular Probes) for each of the 3 variants, with wild-type and the p.R139C variant included as controls. The inflection point of each curve indicates the temperature for protein unfolding and is thus a measurement of stability. RFU, relative fluorescence unit.
Next, we characterized the enzymatic activity of the 3 variant NUDT15 proteins (Figure 1C). Compared with wild-type NUDT15 that efficiently converts TGTP to TGMP (Vmax/Km [catalytic efficiency, pmol/μg enzyme/min/μM TGTP] of 85.6 ± 37.2), all 3 novel variant proteins showed loss of nucleotide diphophatase activity: the p.K35E variant had a Vmax/Km of 38.2 ± 12.0, whereas activity could not be detected for the p.R34T and p.G17_V18del variants. A thermal stability assay showed reduced (the temperature midpoint for the protein unfolding transition) values for all 3 variant NUDT15 proteins (48.7 ± 0.04, 47.7 ± 0.03, 31.3 ± 0.2, and 38.4 ± 0.1°C for p.R34T, p.K35E, p.G17_V18del, and p.R139C, respectively) compared with wild-type NUDT15 (54.8 ± 0.1°C; Figure 1D). In fact, the p.G17_V18del variant was even more unstable than the p.R139C variant, which had the most severe loss of function in our previous report,8 raising the possibility that p.G17_V18del is a very high-risk variant for profound toxicity in patients. These results were in line with the reduced MP tolerance observed in patients (Table 1), providing functional evidence for genotype-guided thiopurine dose reductions.
Although there is a growing appreciation of NUDT15 variants as the primary genetic cause for thiopurine toxicity in Asian patients,7-9,16-22 the importance of this gene in thiopurine pharmacogenetics was less understood in European or African patients, probably due to the rarity of the main p.R139C risk variants in these populations. As the first NUDT15 risk variant identified in non-Asian and non-Hispanic patients with ALL, characterization of the p.G17_V18del variant extends the importance of NUDT15 polymorphism to additional populations. Although still relatively rare (0.26% and 0.05% in the Exome Aggregation Consortium database of 36 677 European and 5203 Africans, respectively; supplemental Figure 1; supplemental Table 1), this variant resulted in highly damaging effects on NUDT15 activity and therefore predisposition to severe toxicity in patients. Interestingly, red blood cell thioguanine nucleotides in the patient with the p.G17_V18del variant (subject 5 in Table 1) were comparable to those from ALL patients with different genotypes at the p.R139C variant after normalization to MP dosage7,23,24 (supplemental Figure 2), which is consistent with our recent report that total thioguanine nucleotides are not influenced by NUDT15 genotype and thus may not be an informative pharmacological marker for toxicity in NUDT15-deficient patients.25 Our findings indicate that NUDT15-related toxicity can occur in all major race/ethnic groups, and thus NUDT15-guided thiopurine dosing has clinical implication across diverse populations. However, it should be noted that the 3 novel variants reported in this article were rare, and our sample size was limited for definitive analyses of their exact effects on MP intolerance, even though our functional characterization provides strong evidence corroborating their association with MP toxicity. Surveying the entire Exome Aggregation Consortium database of whole-exome sequences of 60 706 individuals, there are 85 coding nonsynonymous NUDT15 variants, the vast majority of which are rare. Systematic functional assays of these variants are warranted in the future to understand and anticipate their potential effects on MP toxicity. Because our current study focused only on NUDT15 and TPMT, it is also possible that novel risk variants in other genes might have been missed. In fact, a significant proportion of interpatient variability in thiopurine intolerance remains unexplained by NUDT15 or TPMT,8 suggesting that genome-wide sequencing studies are needed in the future to discover additional genetic risk factors.
The NUDT15 protein has 3 glycine-valine (G-V) repeats at amino acid positions from 13 to 18, and this region of the gene appears to be particularly vulnerable to genetic polymorphism (Figure 1B). Either amino acid insertion or deletion at this position resulted in significant perturbation of NUDT15 activity. In fact, this G-V string is located in the NUDIX fold, a key catalytic motif for the diphosphatase activity,10,26,27 with these G-V repeats directly facing the substrate binding pocket (Figure 1B),10,27 suggesting that the amino acid substitutions at this position might negatively affect catalytic activity by altering the conformation of the substrate binding pocket.
In conclusion, our findings provide further rationale for preemptive NUDT15 genotyping to enable individualized thiopurine dose reduction and safer use of this important antileukemic agent.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and parents who participated in the clinical trials. This work was supported by the National Institutes of Health (National Institute of General Medical Sciences grants GM118578 and GM115279, and National Cancer Institute grant CA021765), the American Lebanese Syrian Associated Charities of St. Jude Children’s Research Hospital, the V Foundation for Cancer Research (T2015-006), the Ministry of Science and Technology, Republic of China (103-2314-B-002-059-MY3), the National Medical Research Council, Singapore (NMRC/CSA/0053/2013), the Children’s Cancer Foundation, and the VIVA Foundation for Children with Cancer. T.M. is supported by the Garwood Fellowship at St. Jude Children’s Research Hospital and the Mie Prefecture Study Abroad Scholarship (Mie, Japan).
Authorship
Contribution: J.J.Y. conceived the research project, collected data, analyzed data, interpreted data, wrote the manuscript, and gave final approval; T.M. performed experiments, collected data, analyzed data, interpreted data, and wrote the manuscript; M.V.R., W.E.E., R.N., T.-N.L., C.L., W.Y., D.-T.L., C.-H.Y., and S.K. collected data, analyzed data, interpreted data, and critically reviewed the manuscript; and Y.-L.Y., H.A., S.J., C.-H.P., and A.E.-J.Y. evaluated patients, collected data, analyzed data, and critically reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jun J. Yang, Department of Pharmaceutical Sciences, St. Jude Children's Research Hospital, MS313, 262 Danny Thomas Pl, Memphis, TN 38105; e-mail: jun.yang@stjude.org.