To the editor:
With the advent of pathway inhibitors, the management of high-risk chronic lymphocytic leukemia (HR-CLL) has dramatically changed during recent years.1-3 This has downscaled the role of allogeneic hematopoietic cell transplantation (allo-HCT) as the formerly most effective treatment of HR-CLL.4-6 The purpose of this analysis was to provide the 10-year follow-up of the multicenter CLL3X trial of the German CLL Study Group (GCLLSG) (#EU-20554, #NCT00281983), which evaluated reduced-intensity conditioning allo-HCT in patients with HR-CLL. The aims of this update were (1) to provide overall 10-year survival results; (2) to assess the impact of graft-versus-leukemia (GVL)–mediated clearance of minimal residual disease (MRD) on long-term disease control; (3) to study the outcome of those patients who had survived 6 years relapse-free post–allo-HCT (landmark analysis); and (4) to study survival after post–allo-HCT relapse.
Between 2001 and 2007, CLL3X enrolled 100 patients (median age 53 years; range 27-65 years), of whom 90 patients were allografted with blood stem cells from related (40%) or unrelated donors (60%) using fludarabine alkylator–based reduced-intensity regimens. A total of 24% had refractory disease at allo-HCT, and 35% had a TP53 deletion and/or mutation. Detailed patient characteristics and outcome results including the observation that genetic risk factors such as TP53 lesions had no prognostic impact have been previously reported.7,8 For the present analysis, survival and relapse information was requested for all patients who were not reported as dead at the most recent follow-up analysis8 from participating study sites, except the Canadian site, which was unavailable for follow-up. MRD results were obtained from the GCLLSG central MRD laboratory in Kiel.9
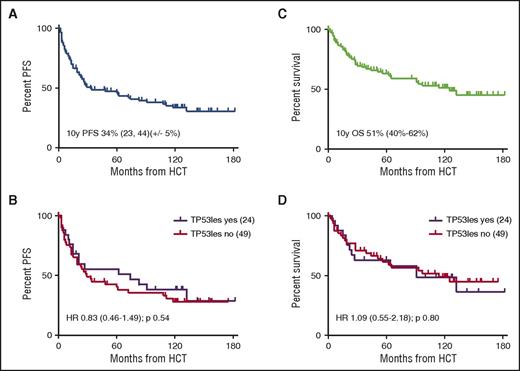
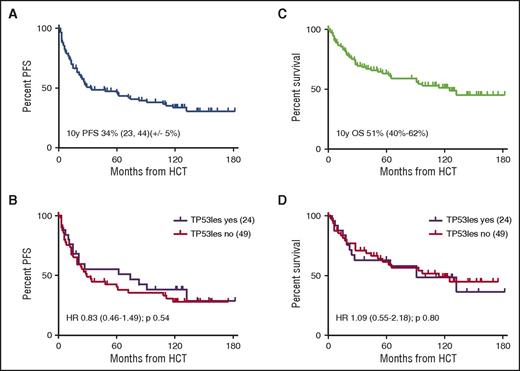
Of the 90 patients transplanted, 37 (41%) had been reported as dead at the 6-year follow-up (17 non–relapse mortality [NRM]; 20 CLL). This included 9 of the 12 patients who had received in vivo T-cell depletion (TCD) with alemtuzumab. Disregarding 9 patients from Canada, status information was sought for the remaining 44 patients, which could be retrieved for 37 of them (84%). Of these 37 patients, 5 had died (3 CLL, 1 chronic graft-versus-host disease [GVHD], and 1 secondary cancer), and 3 had experienced disease recurrence at 6.9, 9.3, and 9.7 years post–allo-HCT, respectively. With a median (range) follow-up of survivors of 9.7 (0.6-15.2) years, 10-year NRM, relapse incidence (REL), progression-free survival (PFS), and overall survival (OS) of all 90 patients allografted was 20% (95% confidence interval [CI], 15-36), 46% (95% CI, 43-67), 34% (95% CI, 23-44), and 51% (95% CI, 40-62), respectively, without significant effects of TP53 lesions on outcome (Figure 1). In contrast, active disease status at HCT and alemtuzumab ex vivo TCD as the most important risk factors identified in the previous analyses retained their significant adverse impact after multivariate Fine and Gray regression modeling (NRM for refractory vs sensitive disease at HCT: hazard ratio [HR], 10.2; 97.5% CI, 3.09-33.4; REL: HR, 0.49; 97.5% CI, 0.18-1.32; NRM for alemtuzumab TCD [yes vs no]: HR, 5.81; 95% CI, 1.79-18.8; REL: HR, 1.13; 95% CI, 0.45-2.84). Ten-year NRM, REL, PFS, and OS for the 59 patients who had sensitive disease at HCT and did not receive alemtuzumab TCD was 9% (95% CI, 2-23), 51% (95% CI, 40-68), 41% (95% CI, 27-54), and 61% (95% CI, 47-75).
PFS and OS of all patients allografted and by TP53 lesion (n = 73). (A-B) PFS of all patients allografted (A) and by TP53 lesion (B) (n = 73). (C-D) OS of all patients allografted (C) and by TP53 lesion (D) (n = 73).
PFS and OS of all patients allografted and by TP53 lesion (n = 73). (A-B) PFS of all patients allografted (A) and by TP53 lesion (B) (n = 73). (C-D) OS of all patients allografted (C) and by TP53 lesion (D) (n = 73).
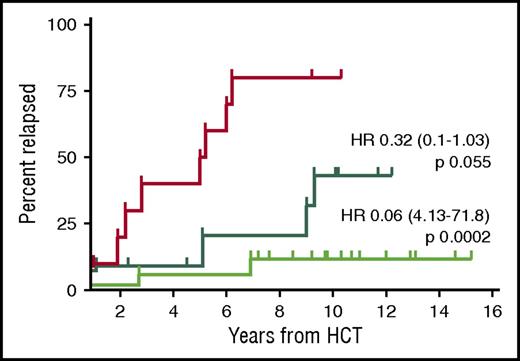
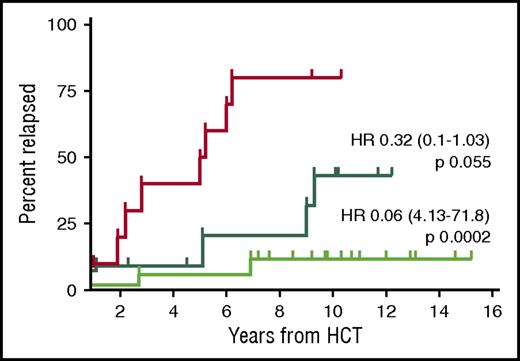
Absence of MRD at the 12-month landmark post–allo-HCT was highly prognostic for a reduced relapse risk (10-year REL 25% vs 80% if MRD was present at the 12-month landmark, P < .0001). The protective effect of MRD negativity at the 12-month landmark was even more pronounced if MRD clearance occurred only after immunosuppression withdrawal, suggesting effective GVL activity (10-year REL 12%) (Figure 2). This observation is in keeping with a previous single-center study reporting long-term MRD-negative disease control in those patients who experienced immune-mediated MRD disappearance.10
Relapse incidence of patients with known MRD status and event-free at 12 months after allo-HCT (n = 38). The red curve shows the relapse incidence of patients who were MRD positive at the 12-month landmark post–allo-HCT (n = 10). The dark green curve shows the relapse incidence of patients who became MRD negative immediately after transplantation and remained so at the 12-month landmark (n = 11). The bright green curve shows the patients who became MRD negative only after immunosuppression tapering and remained so at the 12-month landmark (n = 17). HR, hazard ratio (reference red curve).
Relapse incidence of patients with known MRD status and event-free at 12 months after allo-HCT (n = 38). The red curve shows the relapse incidence of patients who were MRD positive at the 12-month landmark post–allo-HCT (n = 10). The dark green curve shows the relapse incidence of patients who became MRD negative immediately after transplantation and remained so at the 12-month landmark (n = 11). The bright green curve shows the patients who became MRD negative only after immunosuppression tapering and remained so at the 12-month landmark (n = 17). HR, hazard ratio (reference red curve).
The 32 patients who were alive and event-free 6 years after allo-HCT did not differ from the whole trial population in terms of age, pretreatment, fludarabine resistance, and donor source, suggesting that these patients do not represent a positive selection of pretransplant risks. They showed, however, a trend toward a higher frequency of TP53 abnormalities (42% vs 35%) and less often a refractory disease status at allo-HCT (16% vs 24%), but this was not significant.
In these 32 patients, NRM, REL, PFS, and OS 4 years after the 6-year landmark (or 10 years after transplant) was 3.4% (95% CI, 0-10), 18% (95% CI, 4-32), 79% (95% CI, 65-94), and 94% (95% CI, 85-100) with a median (range) follow-up of 4.3 (1.2-9.2) years after the 6-year landmark. Notably, no relapse event occurred beyond 10 years post–allo-HCT. Of the 23 patients who had an observation time of 10 years or longer, MRD results were available for 7 patients at their most recent follow-up and were all negative.
Altogether, 39 of the 90 allografted patients had CLL recurrence after transplant (34 between 2003 and 2010 and 5 from 2011 onwards). While the median survival of those patients who relapsed during the earlier period was 19 months and, thus, in the range of previous reports from the preibrutinib era,10,11 all 5 patients with late relapse are currently alive 4 to 62 months (median 28 months) after the event. Three patients achieved sustained disease control with ibrutinib and one with donor lymphocyte infusion in combination with chemoimmunotherapy. The remaining patient had a very delayed treatment indication and has currently completed chemoimmunotherapy. Course and management of late relapses are shown in supplemental Table 1 (available on the Blood Web site). Thus, although their delayed recurrence pattern may be an indicator of a more indolent disease, the better outcome of the recent relapses probably also reflects the improved rescue options with ibrutinib and other pathway inhibitors that are available nowadays.12
As shown in the original publication and also in other studies, GVL activity is strongly associated with chronic GVHD in CLL.7,10 Accordingly, the 2-year chronic GVHD incidence of 73%7 observed in this trial was unevenly distributed between patients with and without a relapse event. If all patients with at least 1 prior chronic GVHD episode had been excluded, only 5 patients would have arrived event-free at the 6-year landmark, translating in to a 6-year GVHD- and relapse-free survival among all 90 patients of 8% (95% CI, 2% to 14%).
However, it has to be taken into account that chronic GVHD is characterized by a large variance in clinical appearance and severity and, most importantly, can calm down over time. Accordingly, 15 of the 30 patients (50%) included in the 6-year landmark analysis with information available were already off systemic immunosuppression 1 year after transplant. Although a formal analysis of chronic GVHD and quality of life was beyond the scope of this long-term follow-up, this illustrates that the clinical impact of chronic GVHD events may be weighed differently than relapse and NRM events.
In conclusion, this long-term observation of patients allografted in the CLL3X trial indicates that reduced-intensity allo-HCT can provide GVL-mediated sustained disease control in a sizable proportion of patients with HR-CLL independent of TP53 status. Patients who have achieved immune-induced MRD clearance 1 year after allo-HCT have an 87% probability of remaining disease-free for at least 10 years. However, late relapses do occur, and these patients may benefit from strategies involving innovative pathway inhibitors. Taking into account preliminary data suggesting that allo-HCT can be safe and effective in ibrutinib-sensitive but chemoimmunotherapy-refractory patients with HR-CLL,13 the information on long-term results of transplantation provided here may help when counseling patients with advanced CLL about potential treatment options.
The online version of this article contains a data supplement.
Authorship
Contribution: I.K. analyzed data and wrote the paper; S.S., L.U., U.H., A.H., M.H., M.K., N.S., and H.D. designed and performed research; S.D., S.B., M.Z., M.S., J.B., and C.S. performed research; and P.D. designed and performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: I.K. received honoraria from MSD and travel grants from Gilead. S.S. received consultancy fees, honoraria travel grants, and research funding from AbbVie, Boehringer Ingelheim, Amgen, Celgene, Genentech, Genzyme, Gilead, GSK, Janssen, Mundipharma, Novartis, Pharmacyclics, Hoffmann-La Roche, and Sanofi. S.B. received honoraria and research funding from AbbVie; honoraria, travel grants and research funding from Hoffmann-LaRoche; and research funding from Celgene. U.H. received honoraria and financial support for conference participation from Jansen Cilag. M.H. received consultancy and speakers bureau fees from Pharmacyclics, LLC and an AbbVie and speakers bureau fees from Janssen. M.K. received consultancy fees, honoraria, travel grants, and research funding from Gilead and Roche; consultancy fees, honoraria, and travel grants from AbbVie and Janssen-Cilag; research funding from Amgen; and travel grants from Glaxo-SmithKline. P.D. received consultancy fees from Roche and Janssen and consultancy and speakers bureau fees from Novartis and Gilead. The remaining authors declare no competing financial interests.
Correspondence: Isabelle Krämer, University of Heidelberg, Im Neuenheimer Feld 410, Heidelberg 69120, Germany; e-mail: isabelle.kraemer@med.uni-heidelberg.de.