Abstract
Integrin αIIbβ3 is a highly abundant heterodimeric platelet receptor that can transmit information bidirectionally across the plasma membrane, and plays a critical role in hemostasis and thrombosis. Upon platelet activation, inside-out signaling pathways increase the affinity of αIIbβ3 for fibrinogen and other ligands. Ligand binding and integrin clustering subsequently stimulate outside-in signaling, which initiates and amplifies a range of cellular events driving essential platelet processes such as spreading, thrombus consolidation, and clot retraction. Integrin αIIbβ3 has served as an excellent model for the study of integrin biology, and it has become clear that integrin outside-in signaling is highly complex and involves a vast array of enzymes, signaling adaptors, and cytoskeletal components. In this review, we provide a concise but comprehensive overview of αIIbβ3 outside-in signaling, focusing on the key players involved, and how they cooperate to orchestrate this critical aspect of platelet biology. We also discuss gaps in the current understanding of αIIbβ3 outside-in signaling and highlight avenues for future investigation.
Introduction
Integrins are a superfamily of cell-surface receptors, so-named for their properties as integral membrane complexes integrating the extracellular and intracellular environment of cells.1-3 They achieve this through bidirectional signaling, to regulate and respond to the binding of soluble, cell-surface, and extracellular matrix ligands. Integrins are heterodimeric transmembrane glycoprotein complexes comprising noncovalently-bound α and β subunits.4-6 Each subunit consists of a large N-terminal extracellular ectodomain (supplemental Table 1, available on the Blood Web site), a single transmembrane-spanning helix, and a short C-terminal cytoplasmic domain of ∼20 to 60 amino acids.4-8 The latter provides docking sites for multiple signaling and cytoskeletal-associated proteins, which play an essential role in both inside-out and outside-in signaling.8-10 Although often thought of simply as adhesive units, integrins are in fact highly dynamic regulators of cell function. Indeed, they can drive wide-ranging cellular responses including cell spreading, migration, proliferation, and apoptosis, contributing to diverse aspects of organismal physiology, from embryogenesis and wound healing, to immunity and hemostasis.11,12 In contrast, their aberrant function can lead to pathological events such as inflammatory disease, bleeding, and thrombosis.
Integrins are widely distributed throughout mammalian tissues and are maintained in an adhesion-competent state in many cell types. However, for cells such as platelets, it is of critical importance to have more acute and dynamic regulation of integrin affinity and ligand binding via inside-out signaling, to tightly control cellular function. Although we provide a brief introduction to integrins and inside-out signaling, these topics have been the focus of several excellent reviews and we refer the reader to a number of examples.7,13-17 The present review will focus on outside-in signaling by the platelet integrin αIIbβ3. Much of the current understanding of integrin outside-in signaling derives from work on this highly abundant platelet integrin, which drives complex signaling events underlying critical aspects of platelet function.
Platelet integrins
Although platelets express a number of integrins, including α2β1 (collagen), α5β1 (fibronectin), α6β1 (laminin), and αvβ3 (vitronectin), the megakaryocyte- and platelet-specific integrin αIIbβ3 (glycoprotein [GP] IIb-IIIa or CD41/CD61) is the most highly expressed, and is highly affinity regulated.8 Platelets express up to 80 000 αIIbβ3 integrins on their cell surface, and can mobilize further numbers from intracellular stores.18,19 In resting platelets, integrin αIIbβ3 is maintained in a low-affinity state, in which the ectodomain exists in a closed conformation (Figure 1A).20-22 Inside-out signaling to integrins from various activated platelet receptors for soluble ligands (eg, P2Y12, protease-activated receptors [PAR]) or extracellular matrix components (eg, GPIb-IX-V, GPVI) triggers pathways commonly converging on the activation of the small GTPase, Rap1, and the recruitment of the four-point-one, ezrin, radixin, moesin (FERM) domain-containing proteins talin and kindlin.17,23 These proteins bind to the β3 C-terminal tail, to promote domain separation and integrin ectodomain unfolding, converting the integrin to a state capable of high-affinity ligand binding.17,22
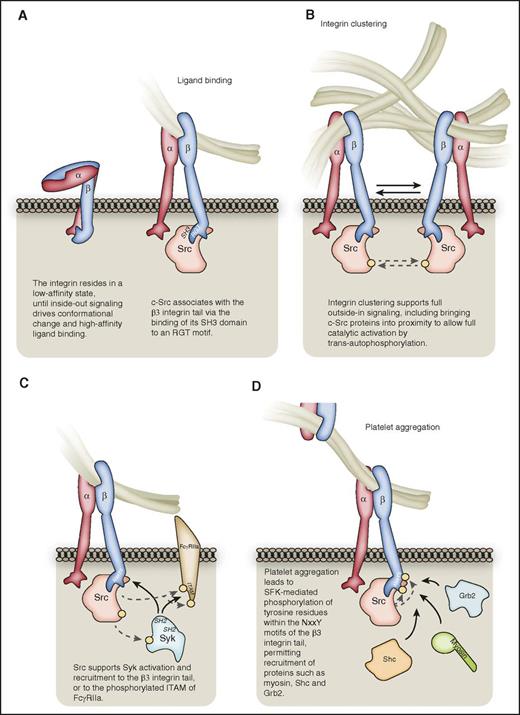
The early stages of αIIbβ3outside-in signaling. (A) Following inside-out signaling, the integrin adopts a conformation that enables it to bind ligands such as fibrinogen with high affinity. c-Src can associate with the RGT motif of the β3-integrin C-terminal tail via its SH3 domain. (B) Integrin clustering supports full c-Src activation, bringing distinct c-Src proteins into proximity for trans-autophosphorylation. For the sake of clarity, all subsequent figures depict nonclustered αIIbβ3. (C) Src supports the activation of Syk kinase, which may bind via its SH2 domains to the β3 C-terminal tail in a manner independent of β3 tyrosine phosphorylation, or to phosphorylated tyrosines within the ITAM of FcγRIIa. (D) αIIbβ3-mediated platelet aggregation leads to SFK-mediated phosphorylation of tyrosine residues within the NxxY motfis of the β3-integrin C-terminal tail, leading to the recruitment of proteins such as Grb2, Shc, and myosin. Hashed lines represent phosphorylation events, denoted on proteins by a yellow circle.
The early stages of αIIbβ3outside-in signaling. (A) Following inside-out signaling, the integrin adopts a conformation that enables it to bind ligands such as fibrinogen with high affinity. c-Src can associate with the RGT motif of the β3-integrin C-terminal tail via its SH3 domain. (B) Integrin clustering supports full c-Src activation, bringing distinct c-Src proteins into proximity for trans-autophosphorylation. For the sake of clarity, all subsequent figures depict nonclustered αIIbβ3. (C) Src supports the activation of Syk kinase, which may bind via its SH2 domains to the β3 C-terminal tail in a manner independent of β3 tyrosine phosphorylation, or to phosphorylated tyrosines within the ITAM of FcγRIIa. (D) αIIbβ3-mediated platelet aggregation leads to SFK-mediated phosphorylation of tyrosine residues within the NxxY motfis of the β3-integrin C-terminal tail, leading to the recruitment of proteins such as Grb2, Shc, and myosin. Hashed lines represent phosphorylation events, denoted on proteins by a yellow circle.
Integrin αIIbβ3 can bind a number of ligands, including fibrinogen, fibrin, von Willebrand factor (VWF), and fibronectin, and ligand binding is influenced by divalent cations.6,22-24 These ligands contain a common Arg-Gly-Asp (RGD) motif, although αIIbβ3 also interacts with other ligand motifs, with the KQAGDV-containing sequence in the fibrinogen γ-chain C terminus being important for platelet aggregation.24-27 Aggregation is mediated by the bridging of fibrinogen between αIIbβ3 on adjacent platelets.28 Much of the current knowledge of αIIbβ3 signaling derives from studies using fibrinogen as a ligand, and the significance of ligand identity on αIIbβ3 outside-in signaling therefore requires further attention. Notably, the precise interplay between fibrinogen and its derivative fibrin as αIIbβ3 ligands in vivo remains unclear, despite an important role for the latter in clot retraction; a recent elegant study revealed qualitative and quantitative differences in the interactions of the two with αIIbβ3, which included contrasting susceptibility to inhibitors.27 Furthermore, both the presentation (eg, soluble vs surface-coated) and density of ligands in the experimental setting can determine outside-in signaling events, possibly reflecting different in vivo contexts of platelet activation, and should be important experimental considerations.27,29,30
Clustering of integrins into hetero-oligomers allows avidity modulation and supports the assembly of the outside-in signalosome.17,31,32 Clustering remains challenging to study experimentally, and current understanding of the mechanisms underlying αIIbβ3 clustering is far from complete. Clustering may be promoted by intracellular protein-protein interactions, including the recruitment of multivalent proteins to the integrin tails, by homomeric transmembrane domain interactions, or by extracellular ligand multivalency, although recent work has suggested αIIbβ3 oligomerization can occur as a result of transmembrane domain separation in the absence of ligand binding.8,17,31,33,34 Regardless, αIIbβ3 clustering complements ligand binding to drive full outside-in signaling, which cannot be induced by monovalent ligands.8,23,33,35 αIIbβ3 outside-in signaling drives processes essential for hemostasis, including platelet spreading, stable thrombus formation, and clot retraction.18,23
The critical importance of αIIbβ3 to platelet function and hemostasis is demonstrated by the serious bleeding disorder caused by the impairment or absence of integrin αIIbβ3 in patients with Glanzmann thrombasthenia.18,36 Mutations in β3 can induce defects in platelet outside-in signaling,37 and patients with a mutation that leads to partial deletion of β3 show constitutive megakaryocyte outside-in signaling, leading to abnormal megakaryocyte spreading on fibrinogen, impaired proplatelet formation, and macrothrombocytopenia.38 Further considerable evidence for the importance of αIIbβ3 outside-in signaling to platelet function comes from genetic mouse models where integrin-regulated proteins have been targeted (Table 1).
The use of αIIbβ3 blockers such as abciximab in percutaneous coronary intervention (PCI) has validated this integrin as a valuable clinical target, and αIIbβ3 has the potential to outcompete other antiplatelet targets in terms of specificity and efficacy of preventing unwanted thrombosis.18 Indeed, the efficacy of therapeutics targeting αIIbβ3 is well established, with a >20% decrease in mortality in randomized studies on patients undergoing PCI.39 However, although the use of radial, instead of femoral, PCI access has reduced access-site bleeding, the use of αIIbβ3 blockers in this context is still associated with a risk of hemorrhagic complications.18,28,40,41 Although the effect is usually less pronounced than directly targeting αIIbβ3, the targeting of certain components of the outside-in pathway downstream of this integrin has shown impairment of thrombosis in experimental animal models (Table 1). Although comparisons between bleeding measurements in rodents and humans should be made with caution,42 the lack of an effect on rodent tail bleeding in these models, including with the use of a peptide that specifically prevents outside-in signaling,43 suggests that targeting this pathway downstream of αIIbβ3 might be a valuable antithrombotic approach to prevent pathological thrombosis without affecting normal hemostasis. Thus, a greater understanding of the components and mechanisms of αIIbβ3 outside-in signaling may provide novel, safer, therapeutic targets.
Outside-in: αIIbβ3 as a signaling receptor
The characterization of integrin outside-in signaling is complicated by the complex nature of the biology itself, and the associated challenges in isolating this pathway experimentally. Indeed, outside-in signaling (1) may be ligand-, integrin-, and cell-type–specific; (2) may not be temporally or spatially distinct from inside-out or other parallel signaling events; (3) may involve a significant number of proteins, that may also be involved in parallel signaling events; and (4) may itself serve as inside-out signaling to further amplify integrin-driven responses. Nevertheless, much progress has been made in developing our understanding of platelet αIIbβ3 outside-in signaling using various approaches,18,44,45 including the use of agents that directly activate (eg, Mn2+, crosslinking antibodies, and domain-disrupting peptides) or inhibit (eg, abciximab, eptifibatide) αIIbβ3, through the identification of αIIb- and β3-binding proteins, and via the study of platelet function. The latter includes assessment of the spreading of purified platelets on immobilized fibrinogen, which reflects a relatively pure readout of αIIbβ3 outside-in signaling, in addition to the study of stable thrombus formation and clot retraction.18,23,28,44 As mentioned, the use of genetic mouse models and platelets from Glanzmann thrombasthenia patients has greatly contributed to this process. An αIIbβ3-expressing CHO cell model has also proved to be a valuable tool, although inevitably some differences exist in the integrin-signaling machinery used by these cells and platelets.46-50
Hence, there is now considerable evidence that activated αIIbβ3 can act as a signal transducer to the cell interior, coupling to intracellular effectors to drive a multitude of outside-in signaling events, ultimately leading to changes in cellular behavior. Platelet spreading, stable thrombus formation, and clot retraction involve significant contributions from the cytoskeletal machinery, and hence outside-in signaling commonly couples αIIbβ3 to actin polymerization, cytoskeletal reorganization, and force sensing/transmission.18,23,51,52 Integrin αIIbβ3 outside-in signaling is also important for megakaryocyte function, including proplatelet formation,38 and holds the potential to drive changes in gene expression in these cells as in other nucleated cell types.18,32 αIIbβ3 outside-in signaling effectors include a host of enzymes, adaptors, and cytoskeletal components; in the following sections, we will highlight the key players driving this complex signaling pathway.
Early αIIbβ3 outside-in signaling events: activation of Src-family kinases and Syk
Src-family kinases
One of the earliest detectable events occurring during integrin outside-in signaling is the tyrosine phosphorylation of specific substrates. Indeed, early reports of integrin αIIbβ3-mediated outside-in signaling followed the observation that platelet aggregation or fibrinogen binding induced the tyrosine phosphorylation of multiple proteins.53,54 The Src family of kinases (SFKs) plays a dominant role in these phosphorylation events, with SFK inhibitors and platelets from mice deficient in multiple SFKs showing impaired protein tyrosine phosphorylation and spreading on fibrinogen.55 Further work using mouse platelets singly or doubly deficient in SFKs has revealed both unique and overlapping roles for individual family members, with c-Src and Lyn proving to be the dominant effectors of αIIbβ3 outside-in driven responses, although Lyn appears to hold both positive and negative roles.11,56,57 Although c-Src, Lyn, and Fyn are the more highly expressed SFKs in platelets,56,58-60 differences in the relative abundance of these kinases between mouse and human platelets mean an element of caution should be applied when interpreting mouse platelets as a model for human platelet SFK signaling; historical SFK inhibitors are also likely to have off-target effects.
Src was originally proposed to be constitutively associated with the β3 C-terminal tail in resting platelets via its Src homology 3 (SH3) domain (Figure 1A), in an inactive conformation likely maintained by C-terminal Src kinase (Csk)-mediated phosphorylation on Tyr 529.55,61 Upon ligand binding to αIIbβ3 and integrin clustering, protein phosphatases such as protein-tyrosine phosphatase 1B (PTP-1B) relieve the inhibitory Src phosphorylation, with dissociation of Csk from β3, permitting Src activation.22,55,61 More recent work demonstrates, however, that c-Src associates with β3 predominantly after platelet activation.62 Furthermore, an interaction of Gα13 with an ExE motif in the β3-integrin tail has been demonstrated as a key early event required for the activation of c-Src to initiate outside-in signaling, a process supported by thrombin signaling.43,63 The receptor-like tyrosine phosphatase CD148 also plays a role in regulating SFK phosphorylation status and activity.64 Deletion of the β3 RGT c-Src–docking sequence protects mice from arterial thrombosis, rendering their platelets defective in outside-in–driven processes, while largely sparing inside-out signaling.65 αIIbβ3 clustering is important for full Src activation, allowing trans-autophosphorylation of the Tyr 418 residue in the Src activation loop (Figure 1B).31
Syk kinase
Integrin-mediated SFK activation leads to the phosphorylation and activation of the tyrosine kinase Syk, and platelets deficient in Syk spread poorly on fibrinogen.55,66 Syk can interact directly with αIIbβ3 via an SH2 domain–mediated interaction with the cytoplasmic tail of β3, although in contrast to Syk’s binding to immunoreceptor tyrosine-based activation motif (ITAM)-containing receptors, this is independent of β3 tyrosine phosphorylation, and of the phosphotyrosine-binding capability of Syk’s SH2 domains (Figure 1C).66,67 In addition, Syk can associate with FcγRIIa following integrin αIIbβ3 activation-dependent phosphorylation of the tyrosine residues within its ITAM, although the low-level expression of this receptor in platelets may limit this mechanism as a general means of αIIbβ3-mediated Syk activation.68
Tyrosine phosphorylation of the β3-integrin tail
Using a mouse model in which platelet outside-in signaling was selectively impaired by mutation of the Tyr 747 and Tyr 759 (human Tyr 773 and Tyr 785) residues within the NxxY motifs of the β3 C-terminal tail, Law et al69 revealed that outside-in signaling is critical for platelet function and hemostasis. Although fibrinogen binding and initial platelet aggregation were preserved, stable aggregate formation and clot retraction were markedly reduced with the mutant platelets, with an associated in vivo bleeding defect. This was likely due to the lack of phosphorylation of Tyr 747 and Tyr 759, as these residues become robustly phosphorylated by SFKs upon platelet aggregation.70,71 Tyrosine phosphorylation of these sites in the β3 tail provides docking sites for adaptor molecules such as SH2 domain-containing-transforming protein C1 (Shc) and growth factor receptor-bound protein 2 (Grb2),72 in addition to the myosin heavy chain (myosin II) (Figure 1D), supporting integrin-cytoskeletal coupling.71 Furthermore, phosphorylation on Tyr 747 of the β3 cytoplasmic tail negatively regulates the binding of talin, whereas phosphorylation on Tyr 759 inhibits β3 tail cleavage by the calcium-regulated protease calpain.23,73 Of note, however, is that many signaling events downstream of β integrins still occur in the absence of β-chain tyrosine phosphorylation and, notably, β2 integrins lack the 2 tyrosine residues present in the β3 cytoplasmic tail.70
Signaling downstream of SFKs and Syk
SFKs phosphorylate a host of signaling and cytoskeletal-associated proteins in platelets, including phospholipase Cγ2 (PLCγ2), focal adhesion kinase (FAK), and adhesion- and degranulation-promoting adaptor protein (ADAP, also known as SLAP-130), leading to their recruitment and/or activation.22,55,74 Syk substrates also include important outside-in effectors, including the Rho guanine nucleotide exchange factors (RhoGEFs) Vav1 and Vav3, and the SH2-containing leukocyte protein of 76 kDa (SLP-76).55,75 The comparable phenotypes of mice lacking such proteins reflect their interconnected roles downstream of αIIbβ3 (Figure 2).
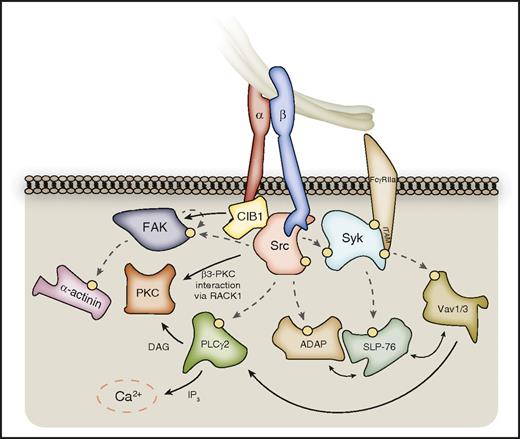
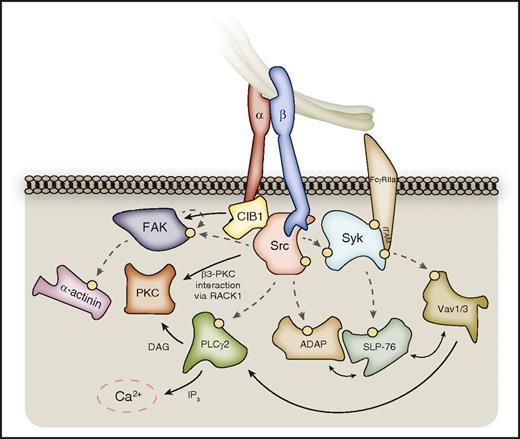
Outside-in signaling downstream of SFKs and Syk. SFKs phosphorylate a host of enzymes and signaling adaptors downstream of activated αIIbβ3, which are important for processes such as platelet spreading. These include PLCγ2, FAK, and ADAP, which in turn further propagate signal transduction. PLCγ2 catalyzes the formation of DAG and IP3 from membrane PtdIns(4,5)P2, leading to PKC activation and calcium liberation, respectively. PKCβ and PKCθ can localize to the β3-integrin tail via RACK1. FAK activation can be supported by CIB-1 bound to the αIIb C-terminal tail, and FAK substrates include the actin-binding protein α-actinin. Syk kinase phosphorylates further downstream targets, including SLP-76, and Vav-family RhoGEFs, which interplay with SFK substrates to propagate outside-in signaling. Hashed lines represent phosphorylation events, denoted on proteins by a yellow circle. Syk may also associate directly with the β3-integrin C-terminal tail.
Outside-in signaling downstream of SFKs and Syk. SFKs phosphorylate a host of enzymes and signaling adaptors downstream of activated αIIbβ3, which are important for processes such as platelet spreading. These include PLCγ2, FAK, and ADAP, which in turn further propagate signal transduction. PLCγ2 catalyzes the formation of DAG and IP3 from membrane PtdIns(4,5)P2, leading to PKC activation and calcium liberation, respectively. PKCβ and PKCθ can localize to the β3-integrin tail via RACK1. FAK activation can be supported by CIB-1 bound to the αIIb C-terminal tail, and FAK substrates include the actin-binding protein α-actinin. Syk kinase phosphorylates further downstream targets, including SLP-76, and Vav-family RhoGEFs, which interplay with SFK substrates to propagate outside-in signaling. Hashed lines represent phosphorylation events, denoted on proteins by a yellow circle. Syk may also associate directly with the β3-integrin C-terminal tail.
PLCγ2 and PKC
Integrin αIIbβ3 ligation results in the SFK-mediated activation of PLCγ2, an enzyme important for αIIbβ3-mediated platelet spreading.76 PLCγ2 activation downstream of αIIbβ3 is also supported by Bruton tyrosine kinase (BTK) and Vav1/3.8,77 This enzyme catalyzes the generation of diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3) from PtdIns(4,5)P2 (Figure 2), with the subsequent downstream activation of protein kinase C (PKC) and increase in intracellular Ca2+, respectively.22,77 The PKC isoforms play various essential roles in platelet function, including inside-out signaling, secretion, and receptor desensitization.22 Of the PKC isoforms, PKCβ and PKCθ associate with β3 integrin cytoplasmic tails (likely via receptor for activated C kinase 1 [RACK1]), and their deficiency results in a platelet-spreading defect on fibrinogen despite normal β3-integrin activation, thus demonstrating a specific role in outside-in signaling.22,78,79 The complete repertoire of PKC substrates in platelets remains unknown and warrants further investigation.80
FAK
FAK was one of the earliest integrin-signaling effectors to be identified and serves as both a tyrosine kinase and a scaffold protein.74 FAK is tyrosine phosphorylated in a manner dependent on agonist and αIIbβ3 signaling, and on actin polymerization, with initial autophosphorylation on Tyr 397 providing a docking site for SFKs that in turn further phosphorylate FAK on multiple residues to promote its kinase activity and enable the recruitment of binding partners.74,81,82 These include Grb2, talin, and paxillin, helping localize FAK to focal adhesion sites, whereas FAK substrates include the actin-binding protein α-actinin.82,83 FAK-deficient platelets show defects in spreading on fibrinogen and in mouse tail rebleeding times, confirming the importance of FAK in platelet-integrin outside-in signaling and thrombus stability.82 Interestingly, megakaryocytes lacking FAK do not show similar defects in αIIbβ3-mediated events, potentially due to greater compensation from its homolog, proline-rich tyrosine kinase 2 (Pyk2).82
SLP-76 and ADAP
SLP-76 deficiency causes mouse fetal hemorrhage with defective platelet spreading on fibrinogen, confirming its importance in αIIbβ3 outside-in signaling.75,84,85 Upon tyrosine phosphorylation, SLP-76 can bind other adaptor proteins, including ADAP, which itself is a target of SFK-mediated phosphorylation.75 Although ADAP has recognized roles in inside-out signaling in platelets, it is also important for outside-in signaling events, including platelet spreading and stable thrombus formation, promoting F-actin assembly necessary for these processes downstream of αIIbβ3 under shear flow.48,52 Both SLP-76 and ADAP interact with the actin-binding vasodilator-stimulated phosphoprotein (VASP).52
Calcium- and integrin-binding protein 1
Calcium- and integrin-binding protein 1 (CIB1) interacts with the cytoplasmic tail of the αIIb subunit and can be a negative regulator of αIIbβ3 activation, while supporting outside-in signaling to mediate platelet spreading on fibrinogen.86-89 Interestingly, recent work has described a role for CIB1 and its interaction with αIIb in the activation of FAK and c-Src, suggesting an important function in the early stages of outside-in signaling.90 The finding that CIB1 also binds to other α subunits suggests it may play a more general role in integrin function.86
Class I phosphoinositide 3-kinase
Although all 4 class I phosphoinositide 3-kinase (PI3K) isoforms have been shown to contribute to platelet function, genetic mouse models and pharmacological inhibitors have revealed PI3Kβ to be particularly important for αIIbβ3 integrin outside-in signaling, including a key role in thrombus stability.91-95 αIIbβ3 activation results in the tyrosine phosphorylation of the E3 protein-ubiquitin ligase c-Cbl and its association with the class I PI3K p85 regulatory subunit in a pathway involving SFKs, Syk, and Pyk2 (Figure 3).45,96,97 In support of this, Pyk2-deficient platelets show a significant defect in αIIbβ3 signaling to the serine/threonine kinase Akt (PKB), in addition to defects in spreading, similar to loss of c-Cbl or PI3Kβ activity.45,97,98 Assessment of PI3K activation downstream of αIIbβ3 is complicated, however, by the importance of released adenosine 5′-diphosphate (ADP) signaling through P2Y12.45 Class I PI3Ks regulate cell function primarily via downstream PtdIns(3,4,5)P3-binding proteins, of which the best characterized is Akt.99 Although the role of the individual isoforms in human platelets is unclear, Akt1 and Akt3 appear important for αIIbβ3-mediated murine platelet spreading with this, and the thrombus instability observed with PI3Kβ deficiency, being dependent on the Akt–glycogen synthase kinase 3 (GSK3) axis.95,100 Similarly, platelets deficient in phosphoinositide-dependent protein kinase 1 (PDK1), a kinase upstream of Akt, show reduced spreading on fibrinogen and clot retraction through dysregulation of Akt and GSK3.101 The PtdIns(3,4,5)P3 binder Ras/Rap GTPase-activating protein (Ras/Rap GAP) Ras GTPase-activating protein 3 (Rasa3) plays a key role in integrin αIIbβ3-mediated cell spreading via regulation of Rap1,47 and other PtdIns(3,4,5)P3-binding small GTPase regulators might also play roles in outside-in signaling through the regulation of Arf and Rho family members (Figure 3).
Outside-in signaling through class I PI3Ks. Class I PI3Kβ is particularly important for thrombus stability, and is activated downstream of αIIbβ3 via a pathway involving the kinases Src, Syk, and Pyk2. This leads to phosphorylation of the E3-protein ubiquitin ligase c-Cbl, which associates with the p85 regulatory subunit of class I PI3K. Activated class I PI3Ks phosphorylate membrane PtdIns(4,5)P2 to form PtdIns(3,4,5)P3, which leads to the recruitment and/or activation of a range of PtdIns(3,4,5)P3-binding proteins. These include kinases such as BTK/Tec, PDK1, and AKT. PtdIns(3,4,5)P3 can also regulate a range of GAPs and GEFs for small GTPases, including RASA3, dedicator of cytokinesis (DOCK) proteins, and Cytohesin-family members. Syk may also associate directly with the β3-integrin C-terminal tail. Hashed lines represent phosphorylation events; yellow circles represent activating phosphorylation; orange circles represent inhibitory phosphorylation.
Outside-in signaling through class I PI3Ks. Class I PI3Kβ is particularly important for thrombus stability, and is activated downstream of αIIbβ3 via a pathway involving the kinases Src, Syk, and Pyk2. This leads to phosphorylation of the E3-protein ubiquitin ligase c-Cbl, which associates with the p85 regulatory subunit of class I PI3K. Activated class I PI3Ks phosphorylate membrane PtdIns(4,5)P2 to form PtdIns(3,4,5)P3, which leads to the recruitment and/or activation of a range of PtdIns(3,4,5)P3-binding proteins. These include kinases such as BTK/Tec, PDK1, and AKT. PtdIns(3,4,5)P3 can also regulate a range of GAPs and GEFs for small GTPases, including RASA3, dedicator of cytokinesis (DOCK) proteins, and Cytohesin-family members. Syk may also associate directly with the β3-integrin C-terminal tail. Hashed lines represent phosphorylation events; yellow circles represent activating phosphorylation; orange circles represent inhibitory phosphorylation.
Rho-family small GTPases
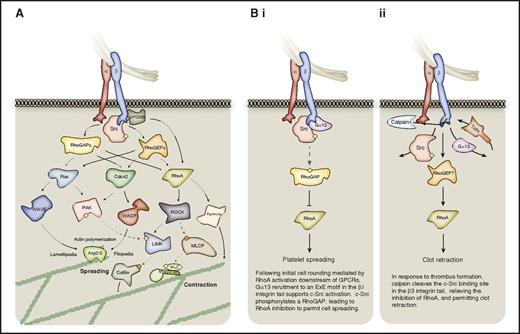
The Rho-family GTPases RhoA, Ras-related C3 botulinum toxin substrate 1 (Rac1), and cell division control protein 42 (Cdc42) are important for global actin dynamics in various cell types and thus provide a route for integrin-mediated changes to the cytoskeleton (Figure 4). RhoA holds a key role in platelet shape change, although its precise role in αIIbβ3 outside-in signaling has been controversial.63,102,103 Activation of a RhoGAP by c-Src in response to Gα13 recruitment to the activated β3-integrin tail has been proposed to mediate the inhibition of RhoA to permit platelet spreading (Figure 4Bi).23,63 In contrast, subsequent thrombus formation and coagulation induces calpain to cleave the c-Src–binding site from the β3-integrin tail, relieving RhoA inhibition to permit clot retraction (Figure 4Bii).23,63,104,105 Accordingly, RhoA-deficient platelets display normal spreading on fibrinogen with only a minor change in morphology, with RhoA being essential for clot retraction, and important for thrombus stability.102 The role of Gα13 in platelet spreading, however, is controversial.43,63,102 Vacuolar protein sorting-associated protein 33B (VPS33B), a member of the Sec1/Munc18 family, binds directly to the integrin β subunit, and was also recently shown to function upstream of Rac1 and RhoA to regulate cell spreading and clot retraction independently of c-Src.106 Active RhoA can signal through Rho-associated kinase (ROCK) activation, which phosphorylates and inactivates myosin light chain (MLC) phosphatase leading to increased MLC phosphorylation, enabling actomyosin contractions.107 RhoA may also regulate microtubule organization via ROCK or formins.102
Outside-in signaling to the actomyosin cytoskeleton via Rho-family small GTPases. The Rho GTPases are particularly important for platelet spreading and retraction. (A) The activation status of the 3 Rho-family small GTPases, Rac, Cdc42, and RhoA is regulated by GAPs and GEFs downstream of activated integrins. When GTP-bound and active, these small GTPases signal to the actomyosin cytoskeleton via multiple effector proteins. Cdc42 and Rac can promote Arp2/3-mediated actin polymerization via WASP and WAVE proteins, respectively, whereas RhoA promotes MLC phosphorylation via ROCK-mediated inhibition of MLC phosphatase (MLCP). These small GTPases may also regulate actin dynamics via proteins such as cofilin, and via formins. (B) In platelets, regulation of RhoA activity coordinates platelet spreading and subsequent clot retraction, as discussed in panels i and ii. Hashed lines represent phosphorylation events; yellow circles represent activating phosphorylation; orange circles represent inhibitory phosphorylation.
Outside-in signaling to the actomyosin cytoskeleton via Rho-family small GTPases. The Rho GTPases are particularly important for platelet spreading and retraction. (A) The activation status of the 3 Rho-family small GTPases, Rac, Cdc42, and RhoA is regulated by GAPs and GEFs downstream of activated integrins. When GTP-bound and active, these small GTPases signal to the actomyosin cytoskeleton via multiple effector proteins. Cdc42 and Rac can promote Arp2/3-mediated actin polymerization via WASP and WAVE proteins, respectively, whereas RhoA promotes MLC phosphorylation via ROCK-mediated inhibition of MLC phosphatase (MLCP). These small GTPases may also regulate actin dynamics via proteins such as cofilin, and via formins. (B) In platelets, regulation of RhoA activity coordinates platelet spreading and subsequent clot retraction, as discussed in panels i and ii. Hashed lines represent phosphorylation events; yellow circles represent activating phosphorylation; orange circles represent inhibitory phosphorylation.
Rac and Cdc42 are well known to regulate formation of lamellipodia and filopodia, respectively, in various cell types.108 Rac1 contributes to lamellipodia formation during the spreading of platelets on fibrinogen, and promotes thrombus stability.109-111 Rac1 also has a role in clot retraction in a MAPK-MLC pathway downstream of αIIbβ3.112 The role of Cdc42 in integrin outside-in signaling in platelets is more controversial, with Cdc42 deficiency in one study reported to have no effect on platelet filopodia formation and spreading on fibrinogen,113 whereas another showed impaired filopodia formation,114 potentially due to the different approaches used to generate mice. Further studies support the idea that the Cdc42–Wiskott-Aldrich syndrome (WAS) protein (WASP) axis is not essential for platelet filopodia formation or spreading.115,116 In contrast to RhoA, the upstream mechanisms of Rac and Cdc42 activation during αIIbβ3 outside-in signaling remain poorly defined and warrant further investigation. Several RhoGEFs and GAPs are present in platelets and are likely to coordinate this process.99 However, in murine platelets, the RhoGEFs Vav1 and Vav3 support αIIbβ3-mediated spreading by regulating PLCγ2 activity in a manner that appears to be independent of Rac.77
Of the many effector molecules downstream of the Rho GTPases, WASP is a major component of αIIbβ3 outside-in signaling, with platelets from WAS patients and WASP-deficient mice showing defects in platelet spreading on fibrinogen, clot retraction, and primary plug stabilization.117 WASP is activated by Cdc42 and partners with and activates actin-related protein complex 2/3 (Arp2/3), supporting the nucleation of actin filaments to initiate formation of the branched actin network, which is important for events like filopodia and lamellipodia formation during platelet spreading. Rac can also activate Arp2/3 through the WASP-family verprolin-homologous (WAVE) 1-3 proteins, although loss of WAVE-1 in platelets did not lead to a defect in lamellipodia formation on fibrinogen.118 Inhibition of Arp2 in platelets was shown to ablate cell spreading.119 Rac1 and Cdc42 may also regulate microtubule dynamics via proteins such as IQ motif containing GTPase-activating protein-1 and cofilin.116
Proteins enabling more direct integrin-cytoskeleton coupling
Although Rho GTPases and other signaling proteins discussed in this article can provide a means for activated integrins to drive actin polymerization and cytoskeletal reorganization, a number of proteins allow more direct integrin-cytoskeletal coupling. The direct force sensing and transmission across the cell membrane that this coupling allows is particularly important for platelet spreading and thrombus stability under shear, as well as for clot retraction.18,51,52 Global approaches have defined core protein components of the integrin adhesome of nucleated cells that enable such coupling, including talin, vinculin, α-actinin, and parvin.32,120-122 The function of many of these has been investigated in platelets, although in some cases their importance appears less pronounced than in other cell types, reflecting differences in the outside-in machinery of anucleate platelets and nucleated migratory cells, and/or robust redundancy in the former.
In addition to its key role in integrin activation,14,16,123 talin can provide a direct linkage between β-integrin cytoplasmic tails and the actin cytoskeleton (Figure 5).32,51,124 There have been contrasting reports regarding the role of talin in αIIbβ3 outside-in signaling,125,126 although recent work suggests that, although not required for the initial outside-signaling responsible for platelet spreading, a later reassociation of talin with β3 is required for clot retraction.43,51 Upon stretch-induced changes in talin, binding sites for vinculin become exposed, and this crosslinker is important for consolidating the integrin-cytoskeletal linkage in nucleated cells.32,120 However, although vinculin may support megakaryocyte membrane cytoskeletal integrity when force is applied to αIIbβ3, assessment of vinculin-deficient platelets suggests it may not have a major role in platelet αIIbβ3 outside-in signaling, with mice only showing a mild hemostasis defect in a tail-bleeding assay.127 This is surprising given vinculin’s abundance in platelets,58,60,128 its presence in actin nodules,124 and its importance for integrin-actin linkage in other cell types,32 and may be due to robust compensatory mechanisms involving other proteins.127 Similarly, α-actinin can consolidate adhesive complexes,32,74 yet its role in αIIbβ3 outside-in signaling remains unclear. α-actinin can associate with αIIbβ3, and appears to hold a role in regulating its affinity.32,83,129 Its tyrosine phosphorylation by FAK in response to platelet αIIbβ3 integrin outside-in signaling may regulate the coupling of αIIbβ3 to actin.8,83,130 The adaptor protein paxillin can make direct contact with α-integrin tails, and serves as a negative regulator of αIIbβ3 inside-out and outside-in signaling.131 Palladin holds a similar negative regulatory role in platelets, with deficient platelets displaying enhanced spreading on fibrinogen, in vivo thrombus formation, clot retraction, and reduced tail-bleeding time.132
Direct αIIbβ3-cytoskeletal coupling. A number of proteins permit direct coupling of integrins to the actin cytoskeleton, which is important in platelets for processes such as clot retraction. However, the current understanding of this coupling is limited in platelets relative to other cell types. Talin can provide a direct link between the β3-integrin C-terminal tail and actin, and has been reported to be important for clot retraction. Stretch-induced changes in talin lead to the exposure of binding sites for vinculin, although the role for this protein in platelet αIIbβ3 signaling may be minimal. Paxillin and α-actinin can associate with the αIIbβ3 C-terminal tails, and may regulate integrin affinity and actin coupling. Kindlin-3 can couple directly to β3 integrins, and to the actin cytoskeleton via the heterotrimeric complex of ILK, PINCH, and Parvin. ILK can itself couple directly to β3 integrins, and also acts as a scaffold to recruit further proteins. Myosin can bind directly to the tyrosine-phosphorylated β3 C-terminal tail. It is important to note that the integrin-binding sites for many of the depicted proteins may overlap (see “Proteins enabling more direct integrin-cytoskeleton coupling” and Figure 6). The αIIbβ3 adhesome is likely to involve a number of further proteins permitting direct coupling of the integrin to the actin cytoskeleton, which are yet to be identified.
Direct αIIbβ3-cytoskeletal coupling. A number of proteins permit direct coupling of integrins to the actin cytoskeleton, which is important in platelets for processes such as clot retraction. However, the current understanding of this coupling is limited in platelets relative to other cell types. Talin can provide a direct link between the β3-integrin C-terminal tail and actin, and has been reported to be important for clot retraction. Stretch-induced changes in talin lead to the exposure of binding sites for vinculin, although the role for this protein in platelet αIIbβ3 signaling may be minimal. Paxillin and α-actinin can associate with the αIIbβ3 C-terminal tails, and may regulate integrin affinity and actin coupling. Kindlin-3 can couple directly to β3 integrins, and to the actin cytoskeleton via the heterotrimeric complex of ILK, PINCH, and Parvin. ILK can itself couple directly to β3 integrins, and also acts as a scaffold to recruit further proteins. Myosin can bind directly to the tyrosine-phosphorylated β3 C-terminal tail. It is important to note that the integrin-binding sites for many of the depicted proteins may overlap (see “Proteins enabling more direct integrin-cytoskeleton coupling” and Figure 6). The αIIbβ3 adhesome is likely to involve a number of further proteins permitting direct coupling of the integrin to the actin cytoskeleton, which are yet to be identified.
Kindlin-3–deficient platelets show defective spreading on fibrinogen in the presence of thrombin, which cannot be rescued with Mn2+, supporting a role in outside-in signaling.133 Kindlins can bind directly to β3 integrins and connect to the actin cytoskeleton via the heterotrimeric complex of integrin-linked kinase (ILK), particularly interesting Cys-His-rich protein-1 (PINCH) and the actin-binding protein parvin (the IPP complex).32 ILK itself can interact directly with β-integrin tails, coupling to actin via parvin, and holds an important scaffolding role as a recruiter of proteins to consolidate integrin signaling and cytoskeletal coupling.32,74 ILK may also stabilize microtubule dynamics by regulating microtubule polarity through an interaction with IQ motif containing GTPase-activating protein-1.120 ILK and the IPP complex support αIIbβ3 activation,134 whereas ILK also supports αIIbβ3-mediated clot retraction.22,135,136 Recruitment of the Arp2/3 proteins to the integrin adhesome via interactions with FAK and vinculin could allow local regulation of actin polymerization, which may also be achieved through α/β-PAK-interactive exchange factor regulation of Arp2/3 via Rac/Cdc42.32,74 As discussed earlier, myosin has also been reported to bind directly to the tyrosine-phosphorylated β3 C-terminus,71 whereas ADAP has an important role in shear flow-induced mechanotransduction, via mechanisms which are not fully clear.52
Further proteins involved in αIIbβ3 outside-in signaling
The preceding sections detail key proteins involved in αIIbβ3 outside-in signaling, but the sheer wealth of work on this topic means that, due to space limitations, it is far from an exhaustive list. Indeed, proteomics and database analyses have recently associated thousands of proteins with integrin adhesive complexes, highlighting the complexity of this signaling network.121,122 Beyond those discussed earlier, a range of further proteins can have either positive or negative roles in αIIbβ3 integrin outside-in signaling, including lymphocyte adaptor protein (Lnk)- and downstream of tyrosine kinase (DOK)-family proteins, respectively (Table 1).137-139 Protein phosphatases contribute to outside-in signaling to regulate clot retraction,140 whereas the PtdIns(3,4,5)P3 phosphatase SH2-containing inositol-5′-phosphatase 1 (SHIP-1) enables αIIbβ3 outside-in signaling to restrain excessive platelet activation.141 Integrins can also crosstalk with other cell-surface proteins/receptors to mediate outside-in signaling, and αIIbβ3 makes functionally important interactions with tetraspanins such as CD151 and tumor-suppressing subchromosomal transferable fragment cDNA 6 (TSSC6).23,142,143 The small GTPase Arf6 can regulate αIIbβ3 trafficking,144 and many further proteins are likely to be involved in this important, yet understudied, aspect of αIIbβ3 biology.
How is outside-in signaling organized in time and space?
A major challenge in the field of integrin signaling is to understand how such a vast array of proteins interact with the integrin cytoplasmic tails, and interplay with one another, in time and space. Integrin-binding proteins may be segregated by preference for either the αIIb- or β3-integrin tail, whereas elegant work has demonstrated that distinct β3 residues can be important to mediate inside-out and outside-in signaling.43,145 However, it is clear that the interaction sites for many proteins involved in proximal integrin signaling overlap or cluster around hotspots in the short β3 cytoplasmic tail (Figure 6).10,120,146 Observations with well-studied integrin-binding proteins, such as talin, kindlin, and src, have revealed that the dynamics of protein interactions with the integrin tails can be regulated by a number of often interconnected factors, including (1) cooperation between integrin-binding proteins; (2) competition between proteins for overlapping or adjacent sites; and (3) the nature of the integrin C-terminal tail, including conformation, phosphorylation status, and proteolytic cleavage. Further related factors will also govern protein recruitment, including (4) the properties of the individual integrin-binding proteins, such as relative expression and affinity for the integrin, and (5) the nature of the stimulus, including integrin ligand identity, density, and shear forces.
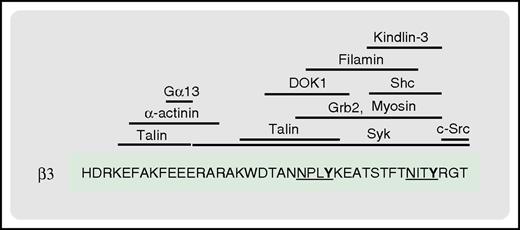
The β3C-terminal tail serves as a docking site for multiple proteins involved in integrin signaling. Shown are the reported binding sites for the indicated proteins. The 2 NxxY motifs of the β3 tail are underlined, with the phosphorylatable tyrosine residues in bold.
The β3C-terminal tail serves as a docking site for multiple proteins involved in integrin signaling. Shown are the reported binding sites for the indicated proteins. The 2 NxxY motifs of the β3 tail are underlined, with the phosphorylatable tyrosine residues in bold.
Talin and kindlin-3 appear to bind cooperatively to the β3-integrin C-terminal tail in a kinetically and thermodynamically linked manner.147 In contrast, competition between talin and filamin A for overlapping binding sites on the β3-integrin tail can regulate integrin activation.148,149 The binding of talin or filamin to the β-integrin C-terminal tail also induces different conformations, which will influence subsequent events.148,150,151 Similarly, as discussed earlier, the dynamic interplay between the binding of talin and Gα13 to the β3-integrin C-terminal tail mediates the switch from inside-out to outside-in signaling, and the transition between platelet spreading and clot retraction.43 SFK-mediated phosphorylation of the NxxY motifs in the β3 tail inhibits the binding of talin, while promoting the association of proteins such as DOK-1, Shc, and Grb2.70,151 Tyrosine phosphorylation also regulates calpain-mediated β3 cleavage,23 whereas phosphorylation of serine and threonine residues in β tails can influence the binding of proteins such as filamin and 14-3-3 proteins.120,146,148 Eventual calpain-mediated cleavage of the β3 tail terminates SFK signaling, whereas calpain can also regulate integrin signaling via the cleavage of key proteins such as talin.23,151
It is clear that αIIbβ3 outside-in signaling cascades are highly ordered, often by sequential phosphorylation events, driving the assembly of the signalosome in a specific and coordinated manner. It is unlikely that all outside-in effectors are required simultaneously, and context-specific roles, localization, and modes of activation will dictate their recruitment. Indeed, evidence in platelets and other cell types demonstrates sequential recruitment and loss of proteins from adhesive/signaling complexes, and it is clear that temporally or functionally distinct stages of outside-in signaling can require different effectors.43,121,122,152,153 Differences in ligand presentation and shear forces can influence αIIbβ3 signaling and protein-protein interactions within the adhesive complex.30,154 Individual outside-in effectors might also shuttle between multiple integrins. Thus, it is likely that, at any one snapshot in time, multiple integrin signalosomes exist containing distinct effectors, depending on the ligand, localization, and timing of activation of individual integrins across the platelet surface. Such observations would align with recent omics data sets, which reveal the αIIb and β3 proteins to be expressed in considerable excess of most downstream signaling proteins in platelets.6,58-60,128 It is therefore clear that the outside-in signalosome is, by necessity, highly dynamic.122,153
Conclusions and future perspective
As discussed, the characterization of integrin outside-in signaling is challenging, particularly due to the component and spatiotemporal overlap with parallel signaling pathways, and the resulting difficulties in isolating specific outside-in events experimentally. Nevertheless, significant gains have been made in identifying many of the key proteins underpinning this complex signaling pathway, and further progress is now needed to understand their intricate behavior and interconnections. It is clear that our understanding of the stoichiometry and spatiotemporal dynamics of integrin outside-in signaling is far from complete, and considerable efforts are needed to overcome the technical challenges in this area. Defining and recapitulating in vivo conditions should remain a major focus, and it will be important to try and replicate the shear forces and complex 3-dimensional milieu of substrates and stimuli experienced by platelets to fully understand the details of αIIbβ3 outside-in signaling in different contexts of physiology and disease. Further understanding of this pathway should come about through continued developments in high-resolution imaging, proteomics, biophysics, and structural biology.
The online version of this article contains a data supplement.
Acknowledgments
The authors thank Alastair Poole, Samantha Moore, and Roger Hunter for helpful discussions. The authors apologize to authors whose work they were unable to cite due to space restrictions.
This work was supported by the British Heart Foundation (grants PG/12/79/29884, PG/13/11/30016, PG/14/3/30565, and RG/15/16/31758).
Authorship
Contribution: T.N.D. wrote and finalized the review and generated the table and figures; M.T.v.d.B. wrote the initial review, edited subsequent versions and contributed to Table 1; and I.H. planned the review, table and figures, and cowrote the review.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for M.T.v.d.B. is Genome Sciences Centre, British Columbia Cancer Agency, Vancouver, BC, Canada.
Correspondence: Ingeborg Hers, School of Physiology, Pharmacology and Neuroscience, University of Bristol, University Walk, Biomedical Sciences Building, Bristol BS8 1TD, United Kingdom; e-mail: i.hers@bris.ac.uk.
References
Author notes
T.N.D., M.T.v.d.B., and I.H. contributed equally to this review.